Abstract
Endometrial carcinoma (EC) is a common female cancer, treated mainly by surgery and adjuvant radiotherapy. Relapse following treatment is associated with increased risk of metastases. Hypoxia, a common microenvironment in solid tumors, correlates with malignant progression, rendering tumors resistant to ionizing therapy. Hence, we assessed here the immunohistochemical expression of hypoxia-inducible factor-1α (HIF-1α) and members of the NF-κB family in 82 primary EC and 10 post-radiation recurrences of EC. Post-radiation recurrences were highly hypoxic, with a higher expression of HIF-1α and also RelA (p65) and p52 when compared with primary EC. We next investigated the effects of hypoxia on EC cell lines. We found that EC cell lines are highly resistant to hypoxia-induced apoptosis. We thus focused on the molecular mechanisms involved in conferring hypoxic cell death resistance. We show that in addition to the classical NF-κB, hypoxia activates the alternative NF-κB pathway. To characterize the upstream kinases involved in the activation of these pathways, we used lentiviral-mediated knockdown and mouse embryonic fibroblasts lacking IKKα and IKKβ kinases. Both IKKα and IKKβ kinases are required for RelA (p65) and p100 accumulation, whereas p52 processing under hypoxia is IKKα dependent. Furthermore, Ishikawa endometrial cell line harboring either RelA (p65) or p52 short-hairpin RNA was sensitive to hypoxia-induced cell death, indicating that, in addition to the known prosurvival role of RelA (p65) under hypoxia, alternative NF-κB pathway also enhances hypoxic survival of EC cells. Interestingly, although HIF-1α controlled classical NF-κB activation pathway and survival under hypoxia through RelA (p65) nuclear accumulation, the alternative pathway was HIF-1α independent. These findings have important clinical implications for the improvement of EC prognosis before radiotherapy.
Similar content being viewed by others
Main
Cellular hypoxia occurs in most, if not all, solid tumors as a consequence of imbalance between oxygen supply and tumor growth. Clinical evidences link tumor hypoxia with cancer progression, metastasis formation and resistance to both radiotherapy and chemotherapy.1, 2 In endometrial carcinoma (EC), hypoxia has been associated with myometrial invasion and development of recurrences after radiotherapy. Hypoxic microenvironment, characterized by acidosis and low nutrient availability, induces both cellular and genomic adaptation mechanisms enabling cancer cells to adapt to stress, promoting thereby more aggressive tumor behavior and resistance to therapy.3 Although genomic adaptation results in genomic instability, allowing outgrowth of resistant cancer clones, cellular adaptation to low oxygen is a process achieved through a number of mechanisms such as angiogenesis and transition from oxidative phosphorylation to glycolysis, and is mainly controlled by hypoxia-inducible factor-1 (HIF-1) transcription factor. Although multiple stimuli have been shown to induce HIF-1 activation, such as bacteria4 or estrogens,5 its accumulation is mainly regulated by hypoxia. HIF-1 is a heterodimeric transcription factor consisting of two subunits HIF-1α and HIF-1β. Although HIF-1β or ARNT is constitutively expressed, HIF-1α's protein stability is oxygen dependant. HIF-1α protein level is maintained low under aerobic conditions. Such control is exerted by a class of prolyl hydroxylases, which in the presence of oxygen hydroxylate HIF-1α at two proline residues (Pro 402 and 564). Hydroxylation of proline residues is a requisite step for recognition and binding to Von Hippel–Lindau protein, which will target HIF-1α for ubiquitination and proteasomal degradation.6 Under hypoxia, HIF-1α is no longer degraded by the proteasome, gets stabilized, shuttles to the nucleus, dimerizes with HIF-1β and initiates transcription of specific genes involved in glycolysis, angiogenesis and oxygen homeostasis.7 HIF-1 induces the expression of glycolytic enzymes and glucose transporters, enabling thereby cancer cell survival by controlling sufficient ATP production. HIF-1α also controls tumor's angiogenesis by inducing the transcription of VEGF,8 thereby increasing oxygen availability. Although HIF-1α appears as the master regulator of oxygen homeostasis under hypoxia, other transcription factors are activated under low oxygen tension such as NF-κB. NF-κB is a family of transcription factors activated by a broad variety of stimulus, such as cytokines, ionizing radiation, chemotherapeutic drugs9 and that control different molecular events, such as stress responses, cell viability, angiogenesis and tumor progression.10 The NF-κB family is composed of five structurally related subunits that belong to two classes: The first class consists of RelA (p65), RelB and c-Rel, synthesized as mature forms, whereas the second class consists of large precursors NF-κB1 (p105) and NF-κB2 (p100), which undergo processing through the ubiquitin proteasome pathway to generate the mature NF-κB subunits p50 and p52, respectively. The NF-κB canonical pathway proceeds through phosphorylation of the IκB kinase (IKK) complex composed of two catalytic subunits (IKKα and IKKβ) and the scaffold essential subunit (IKKγ/NEMO), followed by the phosphorylation of IκB (inhibitor of NF-κB) at serine 32 and 36 residues, which will target IκB proteins for ubiquitin-dependent degradation, allowing freed NF-κB dimers composed of RelA, c-Rel and p50 to translocate to the nucleus.11 Both cytokine-induced IκB degradation and RelA/p50 heterodimers translocation into the nucleus have been shown to depend predominatly on IKKβ.12 The non-canonical or alternative pathway involves NF-κB-inducing kinase (NIK)-mediated activation of IKKα homodimers, which will proteolytically remove the IκB-like C-terminal domain of NF-κB2 (p100), allowing p52, which preferentially dimerizes with RelB, to shuttle to the nucleus.13 The activation of both canonical and non-canonical NF-κB pathways is increased in a wide variety of tumors including EC.14
Elucidation of cell survival pathways under hypoxic conditions may provide insights into the causes of therapy resistance, and thereby open the way to new therapeutic approaches. In the present work, we found an increased expression of HIF-1α and NF-κB family members RelA (p65) and p52 in post-radiation recurrences of EC. Moreover, we show that EC cell lines present a high degree of cell adaptation to hypoxia. By using knockout murine embryonic fibroblasts (MEFs) and lentiviral-mediated knockdown, we show that hypoxia induces NF-κB activity and that this response requires both IKKα and IKKβ kinases. Furthermore, although the classical NF-κB is the major pathway activated under hypoxia, we show for the first time that low oxygen tension induces the activation of the alternative pathway by promoting p100 accumulation, followed by p52 processing. Activation of both classical and alternative NF-κB pathways confers apoptotic resistance to hypoxic cells. As a cross-talk between NF-κB and HIF-1α activation pathways has been reported at different levels, and given the fact that HIF-1α correlates with a subtype of EC aggressiveness,15 we next checked for the importance of HIF-1α in controlling cell survival. We show that HIF-1α is necessary for EC cell survival under hypoxia. HIF-1α controls NF-κB classical pathway signaling, whereas its presence is not required for the activation of the alternative pathway.
MATERIALS AND METHODS
Tissue Microarrays
Two tissue microarrays (TMAs) were constructed. The first one contained samples of 11 post-radiation recurrences of endometrioid carcinomas of the endometrium (EECs), which were obtained from the Departments of Pathology, Massachusetts General Hospital, Boston, MA, and Hospital Universitari Arnau de Vilanova de Lleida, Spain. The second TMA was constructed from paraffin-embedded blocks of 82 EECs, previously evaluated for microsatellite instability, alterations in PTEN, PIK3CA, K-RAS and β-catenin.16 They were obtained from the Departments of Pathology of Hospital Santa Creu i Sant Pau, Barcelona, and Hospital Universitari Arnau de Vilanova de Lleida, Spain, and none of them corresponded to the primary tumors of the same patients with the post-radiation recurrences. The tumors were collected during the period 1996–2007, and they were classified following the most recent World Health Organization criteria. They were surgically staged and graded according to the International Federation of Gynecology and Obstetrics staging and grading systems.17 They included 26 grade I EECs, 35 grade II and 21 grade III EECs. The study was approved by the local ethics committee, and a specific informed consent was used.
A Tissue arrayed device (Beecher Instrument, MD) was used to construct the TMA. Briefly, all the samples were histologically reviewed, and representative areas were marked in the corresponding paraffin blocks. Two selected cylinders (0.6 mm as largest diameter) from two different areas were included in each case. Control normal tissues from the same EC specimens were also included.
Immunohistochemical Study
TMA blocks were sectioned at a thickness of 3 μm, dried for 16 h at 56° before being dewaxed in xylene and rehydrated through a graded ethanol series, and washed with phosphate-buffered saline. Antigen retrieval was achieved by heat treatment in a pressure cooker for 2 min in EDTA (pH: 8.9). Before staining the sections, endogenous peroxidase was blocked. After incubation, the reaction was visualized with the EnVision Detection Kit (DAKO) using diaminobenzidine chromogen as a substrate. Sections were counterstained with hematoxylin. Appropriate positive and negative controls were also tested.
Immunohistochemical results were evaluated by two pathologists by following uniform pre-established criteria. As each TMA included two different tumor cylinders from each case, immunohistochemical evaluation was done after examining both samples.
Antibodies and Reagents
Antibodies to p65, RelB, FADD, Histone H1 and cyclin D1 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to p100/52, caspase 9 and active caspase 3 were from Cell Signaling (Beverly, MA). Anti-HIF-1α and anti-pan ERK antibodies were purchased from BD Biosciences. Anti-caspase 8, anti-IKKα and IKKβ antibodies were from Calbiochem (La Jolla, CA, USA). Monoclonal antibody to tubulin was from Sigma (St Louis, MO). Antibody against lactate dehydrogenase used as a specific marker of cytosolic fraction was obtained from Rockland Immunochemicals (Gilbertsville, PA, USA.). Peroxidase-conjugated anti-mouse and anti-rabbit antibodies were from Amersham Pharmacia (Uppsala, Sweden).
Cell Lines, Culture Conditions and Transfection
The Ishikawa 3-H-12 cell line (IK) was obtained from the American Type Culture Collection (Manassas, VA). KLE cells were a gift from Dr Palacios (Centro Nacional de Investigaciones Oncológicas, CNIO, Madrid). RL-95/2 and HEC-1-A cells were a gift from Dr Reventos (Hospital Vall d'Hebron, Barcelona). Mouse embryonic fibroblasts were a gift from Dr M Karin. Hypoxia (0.2% O2, 94.8% N2 and 5% CO2) was achieved using an In Vivo 2 hypoxic workstation (Ruskin Technologies). All cell lines were grown in Dulbecco's modified Eagle's Medium (Sigma) supplemented with 10% Fetal Bovine Serum (Invitrogen, Carlsbad, CA, USA), 1 mM HEPES (Sigma), 1 mM sodium pyruvate (Sigma), 2 mM L-Glutamine (Sigma) and 1% of penicillin/streptomycin (Sigma) at 37°C with saturating humidity and 5% CO2. When indicated, transfection plasmid constructs were prepared by Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions.
Lentiviral Production and Infection
Lentiviral-based vectors for RNA interference-mediated gene silencing (FSVsi) consisted on an U6 promoter for expression of short-hairpin RNAs (shRNAs) and the Venus variant of YFP under the control of an SV40 promoter for monitoring transduction efficiency. Oligonucleotides to produce plasmid-based shRNA were cloned into the FSVsi vector using AgeI–BamHI restriction sites. Lentiviral particles were produced in 293T human embryonic kidney cells cotransfected by the calcium phosphate method with the above plasmid plus plasmids coding for the envelope and the packaging systems (VSV-G and Δ8.9, respectively).
The 293T cells were allowed to produce lentiviral particles during 3–4 days in the same culture medium of the cultured cells. Culture medium was collected, centrifuged for 10 min at 2500 r.p.m., filtered through a 0.45 μm filter (Millipore, Bedford, MA) and then concentrated by centrifugation through a filter column of 100 kDa (VWR International LLC, West Chester, PE, USA) for 1 h at 4000 r.p.m. Cells were incubated overnight in the presence of medium containing lentiviral particles. After this period, medium was replaced by fresh medium and cells were incubated for two additional days to allow endogenous protein knockdown or protein overexpression.
Sequences of shRNAs
Specific knockdown was achieved using the following sequences for generation of shRNAs:
IKKα: 5′-GCAGGCTCTTTCAGGGACA-3′
IKKβ: 5′-AAAGTGTCAGCTGTATCCT-3′
P65: 5′-ACACTGCCGAGCTCAAGATCT-3′
P100/52: 5′-AAGATGAAGATTGAGCGGCCT -3′
HIF-1α: 5′-CCAGTTATGATTGTGAAGTTA-3′
Plasmids
The luciferase construct containing five NF-κB sites (NF-κB-LUC) (Stratagene, AF 053315) was a gift from Dr Giles Hardingham. Plasmid encoding β-galactosidase was a gift from Mari Carmen Ruiz. Lentiviral luciferase plasmid carrying five NF-κB sites in its promoter was constructed using the Gateway recombination technique. Briefly, attB1 and attB2 flanked primers were designed to amplify a construct containing 5 NF-κB response elements followed by a single luciferase gene from the original vector with the following sequences: attB1NFκBLuc:5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTCATGTCTGGATCCAAGCTAGG-3′ and attB2NFκBLuc:5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTACAATTTGGACTTTCCGCC-3′.
The attB PCR product obtained was purified, and a first recombination step of the PCR product with pDONR vector was performed. A second recombination step was used to transfer the construct to the lentiviral vector pDSL (ATCC). The presence of the attB PCR product in the lentiviral vector was verified by digestion restriction analysis.
Assessment of Apoptosis
IK cells were plated on M4-well plates at 100 × 103 cells/well. Cells were left overnight to allow them to adhere, and then exposed to hypoxic environment for the indicated times. Alternatively in knockdown experiments, cells were plated at 20 × 103 cells/well and infected with the supernatant containing viruses carrying an shRNA. At 3 days after infection, cells were either maintained in normoxia or exposed to hypoxia for additional 24 h.
Hoechst staining was performed by adding Hoechst dye to final concentration of 0.5 mg/ml to each M24 well. Cells were counted under an epifluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Western Blot Analysis
Endometrial adenocarcinoma cell lines were washed with cold PBS and lysed with lysis buffer (2% SDS and 125 mM Tris-HCL (pH 6.8)). Protein concentrations were determined with the Protein Assay Kit (Bio-Rad). Equal amounts of proteins were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore). Membranes were blocked in TBST (20 mM Tris-Hcl (pH 7.4), 150 mM NaCl and 0.1% Tween-20) plus 5% of non-fat milk for 1 h to avoid nonspecific binding and then incubated with the primary antibodies overnight at 4°C. Membranes were then incubated with peroxidase-coupled anti-mouse or anti-rabbit secondary antibodies for 1 h, followed by chemiluminescent detection with ECL Advance (Amersham Pharmacia, Buckinghamshire, UK). Nuclear and cytoplasmic extracts were obtained using NE-PER nuclear and cytoplasmic extraction kit (Pierce).
Cell Cycle Analysis
Analysis of cell cycle distribution was performed, as described.18
5-Bromodeoxyuridine Incorporation
5-Bromodeoxyuridine (BrdU) incorporation assay was performed, as described.18 Nuclei were counterstained with 5 μg/ml Hoechst 33258, and cells were visualized under an epifluorescence microscope (Leica Microsystems).
Luciferase Assay
Luciferase assay was performed, as described.9
RESULTS
Post-Radiation Recurrences of EC Express HIF-1α, RelA (p65) and p52
Hypoxic microenvironments are frequent in solid tumors, and tumor recurrence following irradiation has been associated with the presence of hypoxic cells. The nuclear expression of HIF-1α, and two members of the NF-κB family RelA (p65) and p52, was evaluated in a series of 82 ECs and 11 post-radiation recurrences of EC. There were statistically significant differences in the nuclear expression of HIF-1α, and the two members of the NF-κB family, RelA (p65) and p52, between primary EC and post-radiation recurrences (P=0.004085, P=0.000026 and P=0.023049, respectively). Nuclear HIF-1α, RelA (p65) and p52 immunoexpression was low in primary EC and high in post-radiation recurrences (Table 1). Post-radiation recurrences presented a strong nuclear staining for both HIF-1α and RelA (p65), whereas p52 staining was more moderate (Figure 1).
To check whether there was any association among HIF-1α, RelA and p52 with tumor grade or stage in primary EC, a statistical analysis was carried out in the 82 EECs. No significant association was found between HIF-1α expression and the tumor grade (P=0.25) or stage (P=1). Similar results were obtained with p65 with P-values of 0.22 and 0.42 for grade and stage, respectively. As for p52, its expression correlated with tumor grade (P=0.0166) but not with tumor stage (P=1).
EC Cell Lines Present a High Survival Rate under Hypoxia
To understand the effect of hypoxia on EC cells, we first determined the cell cycle phenotype of four EC cell lines subjected to hypoxic conditions. As described in other cell lines,19 a 24-h hypoxic treatment induced a net proliferative blockade of the four EC cell lines, IK, HEC-1A (HEC), RL-95 (RL) and KLE at the G0/G1 boundary (Supplementary Figure S1). Next, we focused our analysis on IK cell line. As shown in Figure 2a, hypoxic exposure induces HIF-1α accumulation, and leads to a diminution of cell proliferation rate, with a decrease of S-phase cells, of 41.73%, as measured by BrdU incorporation (Figure 2b). Moreover, hypoxic cells exhibited a considerable decrease in cyclin D1 levels compared with normoxic cells (Figure 2a). This is in accordance with the inhibition of G1–S transition known to be mediated mainly by cyclin D family proteins (D1, D2 and D3) and their associated partners, cyclin-dependant kinases.20 Analysis of the sub-G1 content indicates that the four hypoxic endometrial cell lines adapt quite well to hypoxia as the sub-G1 hypoxic DNA content shows a slight increase that does not exceed 2.5% when compared with normoxic conditions (Supplementary Figure S1). Under hypoxic conditions, the number of IK cells displaying nuclear apoptotic morphology, as assessed by Hoechst staining, does not exceed 8% of the total (Figure 2c). Western blot analysis of IK cells exposed to 24 h of hypoxia confirms the apoptotic nature of this cell death, with the activation of executioner caspase 3 (Figure 2d). Overall, these results indicate that under hypoxia, a mere percentage of IK cells undergo apoptosis through the intrinsic pathway, whereas the vast majority of the IK cells, arrested at the G1/S boundary, adapt and survive under low oxygen conditions.
Hypoxia decreases cell proliferation and induces a slight increase in apoptosis in EC cell lines. (a) Ishikawa (IK) cells were incubated for the indicated times under hypoxia, and cell lysates were analyzed by western blot with antibodies to HIF-1α, cyclin D1 and tubulin (left graph). (b) IK cells were incubated for 24 h either under normoxia (21% O2) or under hypoxia (0.2% O2). Pictures show 5-bromodeoxyuridine incorporation and Hoechst 33258 staining (right graph). (c) Quantification of apoptotic nuclei after 24 h of incubation either under normoxic (21% O2) or under hypoxic (0.2% O2) conditions. (d) IK cells were subjected to hypoxia for the indicated times, and cell lysates were analyzed with antibodies to HIF-1α, active caspase 3 and tubulin (right graph).
Hypoxia-Induced NF-κB Nuclear Translocation and Transcriptional Activity Requires the Presence of Both IKKα and IKKβ Kinases
Previous studies have shown that NF-κB pathway is activated under hypoxia.21 However, the mechanisms involved are still controversial. Thus, we next checked for NF-κB activation in our model, and its possible involvement in conferring hypoxic cell death resistance. To address this point, we carried out a NF-κB transcriptional activity assay. IK cells were transfected with NF-κB-dependent luciferase reporter construct and exposed to hypoxic conditions for additional 24 h. TNFα-treated cells served as a positive control. Exposure of IK cells to hypoxia induced a marked increase in NF-κB activity compared with basal luciferase activity under normoxia (Figure 3a). In accordance with this result, immunoblotting of nuclear and cytoplasmic extracts revealed that hypoxia induces RelA (p65) nuclear accumulation in IK cells (Figure 3b). To gain further insight into the mechanisms of NF-κB activation under hypoxia, we explored the requirement of each of the upstream kinases IKKα and IKKβ. Lentiviral delivery of shRNA against IKKα and IKKβ selectively blocked the expression of each protein (Figure 3c). As shown in Figure 3d, NF-κB activation by hypoxia is significantly reduced when either IKKα or IKKβ kinase levels are downregulated, pointing to the requirement of both kinases in hypoxia-mediated NF-κB signaling. Furthermore, overexpression of the mutated form of IκBα, carrying serine to alanine mutations at residues 32 and 36, named SR-IκBα, abrogated hypoxia-induced NF-κB activity (Figure 3d), supporting the notion that IκBα degradation mediates hypoxia-induced NF-κB activation. To check whether the requirement of both IKKα and IKKβ is restricted to endometrial IK cell line, and to eliminate possible nonspecific effects due to the use of shRNA technique, we next studied NF-κB activation response in mouse embryonic fibroblasts derived from either Wt, IKKα and IKKβ knockout mice in response to hypoxia. To carry out NF-κB reporter activity experiment, and as MEF are hardly transfectable, Wt, IKKα and IKKβ knockout MEFs were infected with NF-κB luciferase lentiviral vector. At 3 days after infection, cells were either subjected to hypoxia or treated with TNFα for additional 24 h. In this context, lack of IKKβ caused almost a complete abrogation of either hypoxia- or TNFα-induced NF-κB activation (Figure 3e). However, although genetic ablation of IKKα resulted in a slight decrease of TNFα-induced NF-κB activation, its absence completely abolished NF-κB activation under hypoxia (Figure 3e).
Hypoxia increases NF-κB transcriptional activity and its nuclear translocation through IKKα and IKKβ. (a) IK cells were transfected with the NF-κB luciferase reporter construct and treated with 50 ng/ml TNFα or exposed to hypoxia for 24 h, and cell lysates were assayed for luciferase activity. Results are expressed as relative luciferase units. (b) IK cells were either maintained under normoxia or subjected to hypoxia for 24 h, and nuclear and cytoplasmic lysates were analyzed with RelA (p65) and HIF-1α antibodies. Membranes were also incubated with anti-histone H1 (marker of nuclear fraction) and anti-pan ERK (cytoplasmic fraction) antibodies (right graph). (c) Western blot analysis of IKKα and IKKβ expression in IK cells infected for 3 days with lentiviruses carrying shRNA targeting IKKα or IKKβ. (d) Bar chart showing NF-κB luciferase activity in IK cells infected with lentiviruses carrying shRNA against IKKα, IKKβ or with the lentiviral vector carrying the IκBα superrepressor, which cannot be phosphorylated on serines 32 and 36 (SR-IκBα). After 3 days, cells were transfected with the reporter NF-κB construct together with a plasmid encoding β-galactosidase. After 24 h, cells were either maintained under normoxia (21% O2) or subjected to hypoxia (0.2% O2), and luciferase activity was assayed 24 h later. Results are expressed in relative luciferase units normalized to galactosidase activity. (e) Murine embryonic fibroblasts (MEFs) from Wt, IKKα−/− and IKKβ−/− embryos were infected with NF-κB luciferase lentivirus carrying five κB sites in its promoter. At 3 days after infection, cells were either maintained under normoxia, exposed to hypoxia (0.2% O2) or stimulated with TNFα (50 ng/ml) for additional 24 h before luciferase activity was measured. Results are expressed as relative luciferase units after normalization for protein content.
Hypoxia Induces Alternative NF-κB Activation Through IKKα- and IKKβ-Dependent p100 Accumulation and IKKα-Dependent p52 Processing
Having shown that IKKα controls hypoxia-mediated NF-κB activation, and based on the fact that IKKα is the main component of NF-κB alternative pathway, we sought to investigate the status of the alternative NF-κB pathway under hypoxia. Western blot analysis of total cell lysates of IK cells exposed to hypoxia shows an increase in the expression of p100 and its processed subunit p52, indicating the activation of the alternative NF-κB pathway under this condition (Figure 4a). A concomitant increase in RelB accumulation was observed in response to hypoxic exposure (Figure 4a). Of note, both IKKα and IKKβ subunits accumulate in the nucleus of the hypoxic cells (Figure 4b).
Hypoxia activates the alternative NF-κB pathway through IKKα- and IKKβ-dependent p100 accumulation and IKKα-dependent p52 processing. (a) IK cells were exposed to hypoxia for the indicated times, and cell extracts were tested for p100/52 and RelB proteins by western blot. (b) Murine embryonic fibroblasts (MEFs) from Wt, IKKα−/− and IKKβ−/− embryos were either maintained under normoxia or exposed to hypoxia for 24 h. Nuclear and cytoplasmic fractions were prepared and analyzed for HIF-1α and p100/52 nuclear accumulation. Membranes were also incubated with anti-IKKα, anti-RelA (p65), anti-IKKβ, anti-histone H1 (marker of nuclear fraction) and anti-LDH (cytoplasmic fraction) antibodies.
Next, we wanted to fully characterize the alternative NF-κB pathway induced under hypoxia. Analysis of cytosolic extracts shows that hypoxia stimulated the processing of p100 to p52 in both Wt and IKKβ-deficient MEFs but not in MEFs lacking IKKα (Figure 4b). Analysis of nuclear extracts showed that wild-type MEF exposed to hypoxia accumulated p100 and its processed fragment p52 in their nuclei. Surprisingly, p100 and p52 nuclear accumulation was completely abrogated in both IKKα- and IKKβ-deficient cells (Figure 4b). Overall, these results suggest that although hypoxia-induced processing of p100 to p52 is controlled by IKKα, new synthesis of p100 under hypoxia and its nuclear accumulation together with p52 are controlled by the two kinases IKKα and IKKβ. Similar results were obtained in IK cells when IKKα or IKKβ was silenced by shRNA-based lentiviral vectors (data not shown). Moreover, nuclear translocation of RelA (p65) is abrogated in MEFs lacking either IKKα or IKK β (Figure 4b), confirming the results obtained previously. Of note, both IKKα and IKKβ subunits accumulate in the nucleus of the hypoxic cells (Figure 4b).
RelA (p65) and p52 Promote IK Cellular Survival under Hypoxia
After the identification of both classical and alternative NF-κB pathways activation under hypoxia, and given the central role of NF-κB pathway in regulating cell survival, we next examined the effect of downregulation of both RelA (p65) and p100/52 subunits on IK cell survival under hypoxia. Lentiviral-mediated delivery of shRNA to RelA (p65) subunit achieved an effective depletion of this protein and sensitized IK cells to hypoxic cell death. As shown in Figure 5b, RelA (p65)-deficient hypoxic IK cells displayed a significant increase in the number of cells with apoptotic morphology, assessed by Hoechst staining, together with an increase of caspase 3 processing (Figure 5a). On the other hand, lentiviral delivery of shRNA against p100/52 increased processing of both caspase 9 and 3 in p100/52-deficient IK hypoxic cells compared with their control counterparts (Figure 5c). Cell cycle analysis shows an increase of 4% of the sub-G1 cell fraction in p100/52-deficient IK hypoxic cells compared with the control cells (Figure 5d). Altogether, these results indicate that simultaneous activation of both canonical and alternative NF-κB pathways under hypoxia contribute probably to a better adaptation and survival of endometrial IK cells.
RelA (p65) and p52 promote IK cellular survival under hypoxia. (a) IK cells were infected with lentiviruses carrying shRNA against p65 (RelA) for 3 days and then exposed to 24 h of hypoxia. Cell lysates were subjected to western blot with antibodies to active caspase 3 and HIF-1α. (b) IK cells were infected with lentivirus carrying shRNA to RelA (p65) for 3 days, and then either maintained under normoxia or exposed to 24 h of hypoxia. Then, a quantification of Hoechst-stained apoptotic nuclei was assessed. (c) IK cells were infected with a lentivirus carrying shRNA to p100/52 for 3 days to allow protein knockdown. Whole-cell protein extracts were subjected to western blot analysis to monitor the expression of p100/52 and processed caspase 9 and 3. The blots were subsequently reprobed with anti-tubulin. (d) Flow cytometry analysis and cell cycle distribution. IK cells were infected either with a lentivirus carrying shRNA to p100/52 or with a lentivirus carrying an empty vector for 3 days to allow protein knockdown and then subjected to 24 h of hypoxia or normoxia, after which cell cycle distribution was analyzed.
HIF-1α Controls Hypoxia-mediated NF-κB Activation in IK Cells and Mediates EC Cell Survival under Low Oxygen Tension
HIF-1α transcription factor is known to regulate many of the biological responses under hypoxia, and to promote the expression of a number of survival proteins.22 Having shown that RelA (p65) and p100/52 NF-κB subunits promote cell survival under hypoxia, and given the fact that many studies have demonstrated an interplay between NF-κB and HIF-1α pathways at different levels,4, 23 it was reasonable to test whether HIF-1α regulates NF-κB activity induced under hypoxia in EC cells. To test this hypothesis, we infected IK cells for 3 days with lentiviruses carrying HIF-1α shRNA and subsequently transfected them with the NF-κB luciferase reporter plasmid. As shown in Figure 6a, cells infected with lentiviruses carrying HIF-1α shRNA and exposed to hypoxia significantly reduced the expression of HIF-1α protein. HIF-1α knockdown reduced NF-κB activity in IK hypoxic cells compared with their control counterparts infected with a lentivirus carrying an empty vector. TNFα-stimulated cells served as a positive control for NF-κB activity (Figure 6b). Furthermore, analysis of nuclear extracts shows that RelA nuclear accumulation is partially impaired in IK cells where HIF-1α expression is downregulated (Figure 6a). In contrast, p52 nuclear accumulation under hypoxic conditions was not altered in the absence of HIF-1α (Figure 6a).
HIF-1α controls hypoxia-mediated NF-κB activation and cell survival under low oxygen tension. (a) IK cells were infected with lentiviruses carrying shRNA to either IKKα or HIF-1α for 3 days, then were either maintained under normoxia or exposed to 24 h of hypoxia. Nuclear fractions were prepared and analyzed by western blot using anti-HIF-1α, anti-p65 and anti-p52 antibodies. Histone H1 was used as a nuclear marker. (b) Bar chart showing NF-κB luciferase activity in IK cells infected with lentiviruses carrying shRNA against HIF-1α. After 3 days, cells were cells transfected with the reporter NF-κB construct together with a plasmid encoding β-galactosidase. At 24 h after transfection, cells were either maintained under normoxia (21% O2) or subjected to hypoxia (0.2% O2) or stimulated with TNFα (50 ng/ml), and luciferase activity was assayed 24 h later. Results are expressed in relative luciferase units normalized to galactosidase activity. (c) Quantification of apoptotic nuclei after 24 h of hypoxic exposure. Previously, cells were either infected with an empty lentiviral vector or with a lentiviral vector carrying shRNA to HIF-1α for 3 days. (d) IK cells were either infected with an empty lentiviral vector or with lentiviruses carrying shRNA to HIF-1α for 3 days, and then exposed for the indicated times to hypoxia. Whole-cell lysates were analyzed by western blot with anti-HIF-1α and anti-active caspase 3 antibodies.
As HIF-1α regulates both NF-κB transcriptional activity and RelA nuclear accumulation in IK cells, we next checked whether HIF-1α pathway controls IK cell survival under low oxygen tension. To silence HIF-1α, IK cells were infected with lentiviruses carrying shRNA targeting HIF-1α. At 3 days after infection, cells were subjected to hypoxia or maintained under normoxia for additional 24 h. Interestingly, death resistance of IK endometrial hypoxic cells is completely reversed when HIF-1α expression is silenced. In fact, HIF-1α knockdown caused a significant increase in the number of hypoxic cells displaying apoptotic morphology compared with control cells infected with lentiviruses carrying an empty vector (Figure 6c), and an increase in caspase 3 processing (Figure 6d). This result, together with the reduction of RelA (p65) nuclear accumulation when HIF-1α is silenced, suggests that HIF-1α is a critical upstream regulator of classical NF-κB pathway and cell survival (Figure 7).
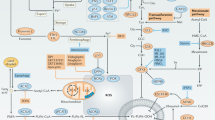
Model for the activation of both canonical and alternative NF-κB under hypoxia. Under low oxygen conditions, stabilized HIF-1α controls canonical NF-κB signaling: Both IKKα and IKKβ kinases are necessary for RelA/p65 nuclear translocation. Hypoxia also mediates the p52-RelB translocation into the nucleus in a HIF-1α-independent manner. Nuclear accumulation of both p100 and p52 requires priming by the canonical pathway through IKKα and IKKβ kinases, whereas p52 processing is IKKα dependent.
DISCUSSION
Cellular hypoxia is considered a major therapeutic challenge, as it decreases radiosensitivity of tumor cells. In the present study, we show that post-radiotherapy recurrences are hypoxic and present higher nuclear expression of RelA (p65) and p52 than primary EC.
Cell survival threshold under hypoxic conditions is cell specific. Hence, although hypoxia triggers cell death in some cell types,24 it promotes cell survival in others.21 We have found that hypoxia decreases cyclin D1 expression and induces G0–G1 phase arrest. The decrease in cyclin D1 expression has been recently attributed to HIF-1α, as HIF-1α has been shown to interact with cyclin D1 promoter.25 Cell cycle arrest may explain the resistance of hypoxic cells to chemotherapeutic drugs that target DNA synthesis in S phase. Thus, a G0/G1 cell cycle arrest may thereby represent a general survival strategy of hypoxic cells, even under chemotherapeutic attacks.25 We show that only a small percentage of EC cells undergo apoptosis under hypoxia. Given the previously reported finding of NF-κB activation under hypoxia,4, 21 our next aim was to investigate the contribution of NF-κB survival pathway in conferring apoptotic cell resistance under hypoxia.
Our results show that hypoxia induced both RelA nuclear accumulation and NF-κB transcriptional activity. However, the upstream pathway leading to NF-κB activation under hypoxia is still under debate. A recent report has attributed to c-Src the capacity to activate NF-κB at tyrosine residues,26 whereas others4 have shown that NF-κB activation under hypoxia is under IKKβ control. By the use of lentiviral-mediated downregulation of IKKα or IKKβ and the mouse embryonic fibroblasts, deficient in either IKKα or IKKβ, we demonstrate that both IKKα and IKKβ subunits of the IKB kinase (IKK) complex are necessary for NF-κB activation under hypoxia. Despite the fact that both kinases have similar primary structure and share 65% identity in their kinase domains,27 it has been shown that IKKα knockout fibroblasts exhibit normal IKK activation and p65/RelA nuclear translocation in response to proinflammatory stimuli, such as LPS, TNFα and IL-1, and a decrease of 50% in total NF-κB DNA-binding activity.12, 28 Recent work of Walmsley et al21 showed an increase of IKKα expression and NF-κB activation under hypoxia, but the interplay linking IKKα to NF-κB was not elucidated.
The fact that IKKα deficiency abrogated completely hypoxia-induced NF-κB activity led us to examine the subcellular distribution of RelA in IKKα-deficient IK cells and MEF knockouts for IKKα. Our results show that IKKα deficiency, like IKKβ deficiency, impairs p65 nuclear translocation under hypoxia. This result indicates that, contrary to proinflammatory cytokine-induced signal, IKKα has a critical role in controlling RelA cellular distribution under hypoxia, and thereby canonical NF-κB pathway. Recent reports have also referred to the requirement of IKKα in some canonical NF-κB signaling pathways.29, 30
As IKKα is known to mediate the alternative NF-κB pathway, we next checked for p52 processing under hypoxia. We show that hypoxia induces p100 accumulation, followed by its processing to p52 in both EC cells and MEFs. This is in accordance with the results published by Mordmuller et al,31 who describe that only newly synthesized p100 undergoes processing generating nuclear p52. Furthermore, p100 processing is a tightly controlled event, mediated by the NIK. NIK functions at the same time as a docking protein, recruiting IKKα to p100,32 and as an IKKα-activating kinase. Activated IKKα in turn phosphorylates p100, promoting its ubiquitination and its processing to p52.33 P100 processing to p52 is activated by a subset of stimuli such as lymphotoxinβ,34 CD40 ligand35 and B-cell-activating factor.36 Furthermore, recent publications have shown that both active Stat337 and active Akt38 induce p100 processing to p52. Both Akt39 and Stat 340 have been shown to be activated under hypoxia. It is thus plausible that either of these pathways serves as an upstream signal for p100 processing to p52 under low oxygen tension. Furthermore, we demonstrate that under hypoxia, IKKα is responsible of p100 processing to p52. This is in agreement with the published role of IKKα in generating p52, activating thereby the alternative pathway.13 However, our results indicate that both IKKα and IKKβ kinases are needed for p100 accumulation. As our data point to the involvement of both kinases in activating the canonical NF-κB pathway, this new result suggests that the activation of the alternative pathway requires previous NF-κB activation through the canonical pathway. Requirement of canonical NF-κB pathway has been reported for LPS-induced alternative NF-κB pathway activation.31 Both p100 accumulation and p52 generation induced by LPS were lost in cells where the canonical pathway was blocked.31 Another evidence demonstrating the cross-talk between both alternative and classical NF-κB pathways is the data obtained by Sun et al41, who describe that transactivation of p65 stimulates p100 mRNA and protein expression, increasing thereby the pool of p100 available for processing to p52. We propose that hypoxia-mediated alternative NF-κB activity is based on p100 accumulation, a phenomena that is under the control of both IKKα and IKKβ kinases. The p100 nuclear accumulation has been described in other cellular models, where it may regulate NF-κB activity by sequestering NF-κB subunits.42 We further investigated the role of p52 and p65 NF-κB subunits in promoting cell survival under hypoxia. Silencing either RelA (p65) or p52 rendered IK cells exposed to hypoxic stress more sensitive to apoptotic cell death, highlighting the prosurvival role of both canonical and alternative NF-κB pathways.
Of note, under hypoxia, we observe that IKKα, and to a lesser degree IKKβ, gets accumulated in the nucleus of hypoxic cells. Nuclear accumulation of IKKα has been described after cytokine exposure and has been shown to regulate NF-κB-dependant gene expression through histone phosphorylation.43 IKKα nuclear accumulation has been shown to control prostate cancer metastasis.44 Additional studies should be performed to elucidate the function of IKKα nuclear accumulation under hypoxic conditions.
As HIF-1α has been shown to contribute to NF-κB activation in murine neutrophils,21 we next checked for the contribution of HIF-1α in activating both classical and alternative NF-κB pathways. Although p65 nuclear accumulation and NF-κB transcriptional activity were reduced when HIF-1α was silenced, p52 nuclear accumulation remained unchanged. HIF-1α silencing increased apoptotic cell death under hypoxia, confirming its prosurvival role under hypoxia.
In summary, we have found that hypoxic stress induces the activation of both canonical and alternative NF-κB pathways, with concomitant nuclear accumulation of both p65/RelA and p52 subunits. To our knowledge, our work is the first to present evidence of the activation of the alternative NF-κB pathway under hypoxia. Our data demonstrate that although activation of the canonical pathway is HIF-1α dependent, processing of p52 through the alternative pathway is HIF-1α independent. Activation of these pathways represents probably a strategy used by cancer cells to survive and escape therapies. HIF-1α, RelA (p65) and p52 may thus be considered as potential therapeutic targets for EC.
References
Harris AL . Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38–47.
Nordsmark M, Overgaard M, Overgaard J . Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1996;41:31–39.
Vaupel P, Kelleher DK, Hockel M . Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 2001;28 (2 Suppl 8):29–35.
Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008;453:807–811.
Kazi AA, Koos RD . Estrogen-induced activation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 2007;148:2363–2374.
Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468–472.
Semenza GL . Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol 2000;59:47–53.
Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992;359:843–845.
Dolcet X, Llobet D, Encinas M, et al. Proteasome inhibitors induce death but activate NF-kappaB on endometrial carcinoma cell lines and primary culture explants. J Biol Chem 2006;281:22118–22130.
Ghosh S, May MJ, Kopp EB . NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225–260.
Karin M, Ben-Neriah Y . Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 2000;18:621–663.
Li ZW, Chu W, Hu Y, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med 1999;189:1839–1845.
Senftleben U, Cao Y, Xiao G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001;293:1495–1499.
Pallares J, Martinez-Guitarte JL, Dolcet X, et al. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J Pathol 2004;204:569–577.
Pansare V, Munkarah AR, Schimp V, et al. Increased expression of hypoxia-inducible factor 1alpha in type I and type II endometrial carcinomas. Mod Pathol 2007;20:35–43.
Catasus L, Machin P, Matias-Guiu X, et al. Microsatellite instability in endometrial carcinomas: clinicopathologic correlations in a series of 42 cases. Hum Pathol 1998;29:1160–1164.
Creasman W . Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105:109.
Sorolla A, Yeramian A, Dolcet X, et al. Effect of proteasome inhibitors on proliferation and apoptosis of human cutaneous melanoma-derived cell lines. Br J Dermatol 2008;158:496–504.
Goda N, Ryan HE, Khadivi B, et al. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 2003;23:359–369.
Swanton C . Cell-cycle targeted therapies. Lancet Oncol 2004;5:27–36.
Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med 2005;201:105–115.
Liu XH, Yu EZ, Li YY, et al. HIF-1alpha has an anti-apoptotic effect in human airway epithelium that is mediated via Mcl-1 gene expression. J Cell Biochem 2006;97:755–765.
Scortegagna M, Cataisson C, Martin RJ, et al. HIF-1alpha regulates epithelial inflammation by cell autonomous NFkappaB activation and paracrine stromal remodeling. Blood 2008;111:3343–3354.
Rosenbaum DM, Michaelson M, Batter DK, et al. Evidence for hypoxia-induced, programmed cell death of cultured neurons. Ann Neurol 1994;36:864–870.
Wen W, Ding J, Sun W, et al. Suppression of cyclin D1 by hypoxia-inducible factor-1 via direct mechanism inhibits the proliferation and 5-fluorouracil-induced apoptosis of A549 cells. Cancer Res 2010;70:2010–2019.
Lluis JM, Buricchi F, Chiarugi P, et al. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res 2007;67:7368–7377.
Zandi E, Rothwarf DM, Delhase M, et al. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 1997;91:243–252.
Hu Y, Baud V, Delhase M, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science 1999;284:316–320.
Takaesu G, Surabhi RM, Park KJ, et al. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol 2003;326:105–115.
Solt LA, Madge LA, Orange JS, et al. Interleukin-1-induced NF-kappaB activation is NEMO-dependent but does not require IKKbeta. J Biol Chem 2007;282:8724–8733.
Mordmuller B, Krappmann D, Esen M, et al. Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Rep 2003;4:82–87.
Xiao G, Fong A, Sun SC . Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem 2004;279:30099–30105.
Xiao G, Harhaj EW, Sun SC . NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell 2001;7:401–409.
Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 2002;17:525–535.
Coope HJ, Atkinson PG, Huhse B, et al. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J 2002;21:5375–5385.
Caamano JH, Rizzo CA, Durham SK, et al. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med 1998;187:185–196.
Nadiminty N, Lou W, Lee SO, et al. Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci USA 2006;103:7264–7269.
Gustin JA, Korgaonkar CK, Pincheira R, et al. Akt regulates basal and induced processing of NF-kappaB2 (p100) to p52. J Biol Chem 2006;281:16473–16481.
Lee SM, Lee CT, Kim YW, et al. Hypoxia confers protection against apoptosis via PI3K/Akt and ERK pathways in lung cancer cells. Cancer Lett 2006;242:231–238.
Noman MZ, Buart S, Van Pelt J, et al. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol 2009;182:3510–3521.
Sun SC, Ganchi PA, Beraud C, et al. Autoregulation of the NF-kappa B transactivator RelA (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc Natl Acad Sci USA 1994;91:1346–1350.
Lessard L, Saad F, Le Page C, et al. NF-kappaB2 processing and p52 nuclear accumulation after androgenic stimulation of LNCaP prostate cancer cells. Cell Signal 2007;19:1093–1100.
Anest V, Hanson JL, Cogswell PC, et al. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature 2003;423:659–663.
Luo JL, Tan W, Ricono JM, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 2007;446:690–694.
Acknowledgements
We thank Concepció Soler and Jordi Torres for critical reading of the manuscript. We thank Dr Michael Karin for providing IKKα−/− and IKKβ−/− MEFs. This work was supported by grants from RD 06/00/20/134, 2009 SGR 174, Fis PI070276, Ministerio de Educación y Ciencia (Juan de la Cierva Postdoctoral fellowship to AY), the Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo (Postdoctoral fellowship CP05/00028 to XD and Predoctoral fellowships FI05/00191 to DL and FI08/0012 to NE), Fundación Científica AECC, Catalunya contra el Cancer, Lleida (fellowship to AS). Tumor samples were obtained with the support of Xarxa Catalana de Bancs de Tumors, The Tumor Banc Platform of RTICC and RD09/0076/000S9. This work is dedicated to Daniel, Alfonso and Irene.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
Hypoxia renders tumors resistant to ionizing therapy. Under hypoxic conditions, both classical and alternative NF-κB pathways are activated in endometrial carcinoma cells, conferring resistance to apoptosis. Post-radiation recurrences present a higher expression of hypoxia-inducible factor-1α, RelA(p65) and NF-κB subunit p52 when compared with primary endometrial carcinoma, implicating them in resistance to radiotherapy.
Supplementary information
Rights and permissions
About this article
Cite this article
Yeramian, A., Santacana, M., Sorolla, A. et al. Nuclear factor-κB2/p100 promotes endometrial carcinoma cell survival under hypoxia in a HIF-1α independent manner. Lab Invest 91, 859–871 (2011). https://doi.org/10.1038/labinvest.2011.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2011.58
Keywords
This article is cited by
-
Bioluminescence Imaging to Monitor the Effects of the Hsp90 Inhibitor NVP-AUY922 on NF-κB Pathway in Endometrial Cancer
Molecular Imaging and Biology (2016)
-
Endometrial carcinoma: molecular alterations involved in tumor development and progression
Oncogene (2013)
-
Transcriptional upregulation of HIF-1α by NF-κB/p65 and its associations with β-catenin/p300 complexes in endometrial carcinoma cells
Laboratory Investigation (2013)