Abstract
Imidazolium salts (IMSs) are precursors to N-heterocyclic carbenes (NHCs), which are routinely used as ligands or organo-catalysts in synthetic chemistry. We recently identified several IMSs as anti-fibrotic agents in liver fibrosis, which often has a consequence in the oncogenesis of hepatocellular carcinoma (HCC). Here, we investigate the potential anti-tumor property of three IMSs (named IBN-1, IBN-9, and DPIM) in HCC cell lines and in a xenograft mouse model. Our results showed that both IBN-1 and IBN-9 significantly inhibited the cell proliferation and arrested HCC cells in the G1-phase, whereas DPIM did not have any anti-tumor activity. When tested in a Huh7 HCC xenograft mouse model, IBN-1 reduced the tumor volume by 31% (P<0.05), however accompanied by a 9% loss in body weight (P<0.005), suggesting a general toxicity. In contrast, IBN-9 significantly reduced the tumor volume by 45% (P<0.05) and 60% (P<0.01) at doses of 0.6 and 1.5 g/l in drinking water, respectively, without any loss in body weight. Our in vitro and in vivo data suggested that IBN-1 and IBN-9 inhibited the growth of HCC by suppressing the expression of Survivin and Cyclin-dependent kinases. The current study provides a proof of concept for using the metal-free IMSs to develop novel anti-cancer agents.
Similar content being viewed by others
Main
Hepatocellular carcinoma (HCC) is the fifth most common cancer and third most frequent cause of death of malignant deaths worldwide, with >600 000 new cases diagnosed each year.1 HCC develops in a setting of liver cellular injury, often accompanied with inflammation, hepatocyte regeneration, extracellular matrix protein deposition, and fibrosis/cirrhosis.2 After several decades of intense research and development, the only FDA-approved drug for the management of late stage HCC so far is a small compound multi-kinase inhibitor (Sorafenib), which prolongs the life of advanced HCC patients by ∼3 months, but with significant side effects including diarrhea, weight loss, hand-foot skin reaction, and hypophosphatemia.3
Survivin is a member of the IAP (inhibitors of apoptosis proteins) family and is generally considered to be an inhibitor of apoptosis. It expresses in a number of cell types during development, but not in healthy differentiated cells, therefore in general, it is considered to be a good molecular target for developing anti-cancer therapy.4 So far, a small molecule YM155 has been developed as a Survivin suppressant in a preclinical model for prostate cancer5 and in a clinical setting for a number of advanced solid tumors, exclusive of HCC.6 Furthermore, anti-sense oligonucleotides for colorectal cancer7 and Survivin peptide vaccine for breast cancer patients8 have also been attempted. A decade ago, Survivin was initially found to overexpress in several HCC cell lines and in a small-sized clinical HCC samples,9 which was subsequently confirmed in an independent study.10 Mechanistic studies revealed that Survivin is not only important in regulating apoptosis, but also pivotal in initializing cell cycle by interacting with Cdk4 and p21 in HCC cells.9 Overexpressing Survivin in several HCC lines (HepG2, Huh7, SK-Hep1, and HLE) all led to an increase in cell cycling, accompanied by a decrease in the number of cells in the G1-phase.9 The observations suggest Survivin as an ideal target for developing anti-HCC agents.
In addition to Survivin, Cyclin-dependent kinases (Cdks) have been investigated as a target for anti-cancer agents for anti-HCC therapies. In particular, the Cyclin D1–Cdk4 complex, which is dispensable for normal development of mammary gland, but is required for the development of mammary malignancy and has become an attractive target for cancer therapy.11 Recently, a small molecule inhibitor (BS-181) for the Cdk-activating kinase (CAK) has been identified with IC50 of 21 nM for the Cdk7 in a cell-free environment, and a cellular IC50 in the range of 15–30 μM for multiple breast cancer cell lines and a HCC line HepG2. When tested in a breast cancer xenograft mouse model with MCF-7, the compound was found to inhibit the growth of tumor mass by 50% after 2 weeks of treatment (i.p., at 20 mg/kg/day).12 To our knowledge, no success has been reported for targeting HCC based on Survivin or Cdk suppression alone, not to mention a strategy for simultaneously targeting both Survivin and Cdk in HCC.
Imidazolium salts (IMSs) are precursors to N-heterocyclic carbenes (NHCs), which are routinely used as ligands or organo-catalysts in synthetic chemistry. We recently showed that a novel class of IMSs are anti-oxidative in vitro13 and anti-fibrotic in vivo in hepatic stellate cells (HSCs),14 which is a major stromal cell type contributing to fibrosis15 and carcinogenesis16 during liver injury. In fact, an anti-fibrotic compound (Tranilast) has been explored for its potential as an anti-cancer agent.17 In this study, we evaluated the anti-HCC potential of three IMSs (IBN-1, IBN-9, and DPIM) and investigated the possible mechanisms of action.
MATERIALS AND METHODS
Compounds and Cell Culture
The chemical structures, names, and molecular weights for IBN-1, IBN-9, and DPIM were depicted in Figure 1. IBN-1 and IBN-9 (both 99% purity) were synthesized as previously described,18 and DPIM (96% purity) was purchased from Sigma-Aldrich Chemicals (St Louis, MO, USA). All three compounds are soluble in water or DMSO. Liver cancer cell lines (HLE, Huh7, Hep3B, HepG2) were purchased from Health Science Research Resource Bank, Japan. Normal liver (THLE-2), lung (IMR90), and breast MCF-10 cell lines were purchased from American Type Culture Collection (ATCC, VA, USA). All these cell lines were routinely cultured in DMEM (Invitrogen, CA, USA) supplemented with 10% fetal bovine serum and 1% of mixture of penicillin and stretomycin in a humidified CO2 incubator at 37 °C, and split at 1:4 ratio by trysinization at confluence.
Cell Proliferation Assay, Immunocytochemistry, and High Content Screening
Cell proliferation assay and immunocytochemistry were performed as previously described.14 The high content screening (HCS) and imaging (Figure 4a and b) were acquired on the ArrayScan VTI HCS reader (Cellomics, Pittsburgh, PA, USA) using antibody against Survivin (Lab Vision, Fremont, CA, USA).
Cell-Cycle Analysis
Cells initially seeded in the regular medium on 10 cm dishes for 24 h were synchronized with the serum-free DMEM medium for additional 24 h. The cells were then treated with IBN-1 (10 or 100 μM) or IBN-9 (120 μM) for 24 h prior to harvest by trypsinization. After a fixation in 70% ethanol for 2 h, the cells were resuspended in propidium iodide (3 μM) staining solution containing RNase A. The DNA content and the cell-cycle distribution of cancer cells were measured with a flow cytometer and quantified with the CellQuest software (FACSCalibur, BD Biosciences, NJ, USA). These experiments were repeated three times for statistical analysis.
TUNEL and Immunohistochemistry
The tumors were collected and fixed in 4% paraformaldehyde (in 1 × PBS, pH 7.4) for overnight. After fixation, the tumors were embedded in paraffin and sectioned in 5 μm thickness. TUNEL staining was performed using TdT In Situ Apoptosis Detection Kit (TA4627, R&D System) according to its standard protocol. For immunohistochemistry, tissue sections were deparaffinized with xylene, rehydrated in a series of descending ethanol concentrations and microwaved in antigen retrieval buffer (Tris-EDTA, pH 9.0). The sections were then permeabilized with 0.5% Triton X-100 and blocked in PBS containing 10% horse serum. The slides were incubated with rabbit anti-human Ki67 antibody (1:250, AB833, Abcam) overnight at 4°C. After rinsing with PBS three times, the slides were incubated with donkey anti-rabbit Alexa Fluo 488 (1:250, Molecular probes, Invitrogen) for 1 h at room temperature. DAPI was used for counterstaining of cell nuclei. The slides were rinsed with PBS three times and mounted with VECTASHIELD Fluorescent Mounting Media (H-1000, Vector Labs). The tissues were examined and photographed using Leica DM IRB inverted microscope.
Western Blot
Protein extraction and blotting were done similarly as previously reported.19 The membrane was then probed with primary antibodies against cIAP, xIAP (R&D Systems, Minneapolis, MN, USA), Survivin, p53, Mdm2, Cdk2, Cdk4, Cdk6 (Santa Cruz Biotechnology, CA, USA), Cdc2 (Cdk1), Cyclin B, D, E, pRB, E2F1, Caspase-3, -9, PARP, Bcl-2, Bax (Cell Signaling Technology, MA, USA), and β-actin (Sigma, USA), and detected by horseradish peroxidase-conjugated secondary antibody (GE Healthcare, UK). Protein bands were recorded on X-ray films by reacting with ECL chemiluminescence reagents (Amersham Biosciences, NJ, USA).
HCC Xenograft Model
Balb/c nude mice, 5–6 weeks old, were inoculated with 1 × 107 Huh7 cells in 0.2 ml volume of Matrigel/DMEM mix. Since not every mouse developed tumor after the inoculation of HCC cells, only those with visible tumors (∼50 mm3 in volume; about 8 weeks after inoculation) were used for subsequent experiment. The tumor-bearing mice were randomly divided to the control and the treatment group (N=8), respectively. Mice in the treatment group had free access to drinking water containing IBN-1 (2 g/l) or IBN-9 (0.6 or 1.5 g/l), while mice in the control group had free access to water only. The compound treatment lasted for 3 weeks, with a weekly change of compound solution. On average, each mouse consumed about 4 ml of water or water containing IBN-1 or IBN-9 daily. The tumor size was measured weekly, and the tumor volume was calculated using the formula: 0.52 × width2 × length.
RESULTS
IBN-1 and IBN-9 Arrested HCC Cells in G1-Phase
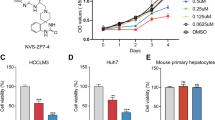
The IC50 values for IBN-1 and IBN-9 in the HCC cell line HLE (day 3) were estimated to be 100 and 120 μM, respectively, which was about one fold higher than that of 5-fluorouracil (5-Fu). Similar values were obtained with the two compounds for three HCC lines (Huh7, Hep3B, and HepG2) regardless of their p53 status. Notably, IBN-1 and IBN-9 did not significantly inhibit the proliferation of non-cancerous liver (THLE-2), breast (MCF-10), and lung (IMR90) cell lines and, hence no reliable IC50 values could be obtained up to the mM range. DPIM did not inhibit any of the four HCC cell lines (HLE, Huh7, Hep3B, and HepG2), even when measured in the mM range. Our additional data also indicated that IBN-1 and IBN-9 lacked significant cytotoxicity, as assayed for LDH leakage in HLE and HepG2 cells using a concentration up to 2.5 mM for 24 h (data not shown). These preliminary observations suggested that the IBN serial compounds were relatively non-cytotoxic, and the anti-proliferative property of the compounds may be due to cell-cycle arrest. To investigate this possibility, HLE cells treated with IBN-1 (10 or 100 μM for 24 h) and Huh7 cells with IBN-9 (120 μM for 24 h) were harvested for cell-cycle analysis with flow cytometry. The results showed that IBN-1 (100 μM) significantly increased the percentage of cells in the G1-phase by approximately 14 percentage points (Figure 2a). A similar G1-arresting efficiency was observed for IBN-9 (Figure 2b).
IBN-1 and IBN-9 arrested HCC cells in G1-phase. (a) Treatment of HLE cells with IBN-1 (10 or 100 μM for 24 h) arrested cells in the G1-phase. Notably, the number of cells in the G1-phase increased from 43 to 58%, with a significant concurrent decrease in the number of cells in the S-phase. (b) Treatment of Huh7 cells with IBN-9 (120 μM for 24 h) yielded similar results. All results were expressed as mean±s.e.m. of three experiments. *P<0.05, **P<0.01 and ***P<0.001.
IBN-1 and IBN-9 Suppressed Expression of Survivin and Cdks
Given the importance of Survivin as a novel regulator in multiple signaling pathways including cell cycle and apoptosis,4 we investigated whether the IMS compounds would have any influence on the expression of Survivin, Cdks and other proteins relevant to cell cycling. We treated four HCC cell lines with three compounds (IBN-1, IBN-9, and DPIM) at 10 or 100 μM for 24 h and immunoblotted the total cellular proteins to look for changes. In the HLE cells, both IBN-1 and IBN-9 dose dependently inhibited the expression levels of Survivin and Cdk4, respectively (Figure 3a). In addition, IBN-1 inhibited Cyclin D1, an interacting partner of Cdk4 and Cdk6 (Figure 3b). In a sharp contrast, DPIM did not alter the expression of Survivin nor any Cdks. In the Huh7 cells, both IBN-1 and IBN-9 also consistently inhibited the expression of Survivin (Figure 3c). At a slightly higher concentration (160 μM, 24 h), IBN-9 inhibited the expression of Cdk2, Cdk4, Cyclin D1, Cyclin D3, and its downstream targets RB and E2F1 (Figure 3d). In contrast, DPIM had no effect on the expression of Survivin nor Cdks/Cyclins. In the Hep3B and HepG2 cells, a variable susceptibility to the compounds was noted in terms of Survivin suppression, for example, from a very weak suppression in the Hep3B (Supplementary Figure S1A) to the very strong one in the HepG2 (Supplementary Figure S1B). To test the specificity of Survivin suppression, we treated the HLE (for 72 h) and HepG2 (24 h) cells with higher concentrations of IBN-1 (up to 1000 μM) and immunoblotted for members of the IAP family (Survivin, xIAP, and cIAP). The results showed no non-specific inhibition of xIAP or cIAP even at 1000 μM (Supplementary Figure S1C and D).
IBN-1 and IBN-9, but not DPIM, suppressed the expression of survivin and Cdk4 in HCC cell lines. Both IBN-1 and IBN-9 (10 and 100 μM, 24 h) inhibited the protein expression of survivin and Cdk4 (a) and Cyclin D1 (b) in the HLE cells. Similarly IBN-1 and IBN-9 (10 and 100 μM, 24 h) also inhibited the protein expression of survivin (c) in the Huh7 cells. At a slightly higher concentration (160 μM, 24 h), IBN-9 not only suppressed Cdk4, but also its binding partner Cyclin Ds and their downstream targets pBR and E2F1 (d), which would help to arrest cells in the G1-phase. Finally, DPIM did not suppress survivin nor any Cdks in both cell lines.
In addition to its expression level, the subcellular localization of Survivin may be critical to both mitosis and carcinogenesis,20 particularly a majority of human HCC tissues displayed a predominantly nuclear staining for Survivin.9, 10 To survey the Survivin cellular distribution in the HLE cells, an proximately 5000 unsynchronized cells stained with Survivin antibody were scanned on the HC ArrayScan VTI HCS reader, and the representative image (Figure 4a) indicated a mix of nuclear (arrow) and cytoplasmic (dotted arrows) staining for Survivin. When the cells were treated with IBN-1 (1000 μM for 24 h), the Survivin was found to translocate from the nucleus to the cytoplasm (Figure 4b). Similarly when the unsynchronized HLE cells were examined with immunocytochemistry, pan-cellular Survivin staining was noted. In addition, an intense Survivin assembly with α-tubulin in anaphase (Figure 4c, empty arrowheads) and with the midbody spindle fibers in late telophase (Figure 4c, arrowheads) was seen in late mitotic cells. Of a great interest, when the cells were treated with IBN-9 for 24 h, all the Survivin assemblies were found to be completely abolished (Figure 4d).
Effects of IMSs on survivin cellular translocation and assembly during mitosis. (a) Survivin locates in both nucleus (arrows) and cytoplasm (dotted arrows) in the HLE cells. An intense staining for survivin was seen in a cell undergoing through mitosis at the telophase (arrowhead). (b) Treatment with 1000 μM of IBN-1 for 24 h translocated survivin from the nucleus (arrows) to the cytoplasm, presumably de-coupling the survivin–Cdk4 complex and preventing cells from entering the cycling process. (c) Survivin associated with α-tubulin in the anaphase (empty arrowheads) and telophase (arrowheads) is visible in the proliferating Huh7 cells, as revealed by survivin antibody. (d) Treatment with 200 μM of IBN-9 for 24 h completely abolished the survivin assembly and interfered with cell mitosis. (e, f) Images of (c, d) co-stained with DAPI. Note in (e) that the survivin assembly at the telophase (arrowheads) was located in the midbody between two newly formed nucleus in the adjacent daughter cells.
IBN-1 and IBN-9 Induced Caspases, but with Minimal Effect on p53 Signaling
In addition to its critical role in cell cycling (eg, inducing Cdk4), Survivin is also known to induce Bcl-2 and to inhibit Bax expression to resist apoptosis.4 To investigate whether the IMS compounds would have any effect on several key proteins relevant in apoptosis, we treated HLE and Huh7 cells with either IBN-1 or IBN-9 and immunoblotted total cellular protein extracts with various antibodies. The results showed that none of the two compounds had any significant influence on the protein level of p53, MDM2, and AIF (Supplementary Figure S2A–C). Similarly, the two compounds did not significantly alter the protein level for Bcl-2 and Bax families in HLE and HepG2 (Supplementary Figure S3A and B), except a slight induction of Bax by IBN-9 in the Huh7 cells (Supplementary Figure S3C). These results suggested that the two IMS compounds are not naive inducers or genotoxic agents that could trigger p53 signaling. A separate test also confirmed these IMS compounds to be free of genotoxicity (data not shown). Nevertheless, both compounds can induce and cleave procaspases in Huh7 cells (Supplementary Figure S4A and B).
IBN-1 and IBN-9 Reduced the Growth of HCC in a Xenograft Mouse Model
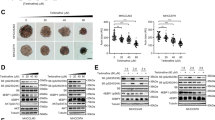
We first tested five HCC cell lines (HepG2, Hep3B, Huh7, PLC, and HLE) for their ability to form HCC in Balb/c nude mice, and found that Huh7 line, which is enriched with cancer stem cells,21, 22 was the most aggressive, and therefore chosen for the xenograft modeling. At the end of the treatment (third week), mice treated with either IBN-1 or IBN-9 had a reduced tumor mass (Figure 5a). Calculation from the measurement indicated a 31% (P<0.05) reduction in tumor volume in mice treated with IBN-1 (2 g/l) (Figure 5b), however, accompanied by a 9% decrease (P<0.005) in body weight (Supplementary Figure S5A). For tumor-bearing mice treated with IBN-9, a 45% (P<0.05) and a 60% (P<0.01) reduction in tumor volume was achieved at the 0.6 and 1.5 g/l dose, respectively (Figure 5c), without any body weight loss (Supplementary Figure S5B).
To assess the effects of IBN-9 on cell proliferation in vivo, immunohistochemistry with Ki67, Survivin and Cdk4 were performed on tumor tissues isolated from mice treated with water alone or with IBN-9 (1.5 g/l). The results showed that IBN-9 treatment significantly suppressed the protein expression of Ki67, Survivin, and Cdk4 (Figure 6). By quantification, the number of Ki67+ cells in the treated tissue was reduced by 66% (P<0.001). In addition, the TUNEL assay results also revealed that IBN-9 triggered apoptosis in tumor tissues (Figure 7).
IBN-9 reduced cell proliferation in HCC tissue. Immunohistochemistry with the Ki67 antibody revealed an active cell proliferation in the tumor tissue (a). Treatment with IBN-9 (1.5 g/l for 21 days) resulted in a 66% (P<0.001) reduction in the number of proliferating cells in the tumor (b). Similarly, the elevated expression of survivin (c) and Cdk4 (e) as seen in the untreated tumor tissues were significantly reduced in tumor tissues treated with IBN-9 (1.5 g/l for 21 days), with (d) being survivin and (f) being Cdk4.
IBN-9 induced apoptosis in HCC tissues. No TUNEL stain was seen in the untreated tumor tissue (a) and the DAPI stain of the same tissue section revealed non-apoptotic nuclei (d). Positive TUNEL staining (green arrows) was visible in tumor tissues from two different tumor-bearing mice treated with IBN-9 (1.5 g/l for 21 days) (b, c). DAPI staining (blue) revealed many condensed and fragmented nuclei (going through apoptosis) (arrow heads in e, f) from the two identical tissue sections corresponding to (b, c). For color see html version.
DISCUSSION
A recent structural analysis on >8000 FDA-approved drugs and drug candidates in the clinical trials showed that IMS is not a major building block for the existing drugs or candidates for any indications, including cancer.23 But in recent years, NHC–metal complex compounds with anti-tumor activity have been reported.24, 25, 26 Most recently, a class of imidazolium-based ionic liquids with a long alkyl chain (≥11) at the N-3 position was shown to kill cancer cells effectively in the NCI60 panel,27 which does not include any HCC cells. Here, we were independently able to show that the structurally distinctive metal-free IMS compounds, for example, IBN-1 and IBN-9, are effective anti-HCC agents in vitro and in vivo. In a preliminary structure–activity relationship (SAR) analysis, we found that the simple DPIM (Figure 1c) lacks anti-tumor activity in HCC lines, whereas IMS compounds with two benzene groups, such as IBN-1, IBN-9, and additional IBN series (IBN-19, IBN-24, IBN-25, and IBN-32; structures not shown), have significant anti-tumor activity.
Anti-cancer compounds based on the inhibition of Survivin5, 28 and Cdks12, 28 have been reported and evaluated in the clinics. Among them, M4N (tetra-O-methyl nordihydroguaiaretic acid) was a compound shown to arrest the transformed C3 cells in G2-phase by simultaneously inhibiting Cdc2 (Cdk1) and Survivin expression.28 On the other hand, it is not certain whether the Survivin suppressant (YM155)5 would also inhibit any Cdks/Cyclins. It is clear from our results that IBN-1 and IBN-9, which are structurally distinctive from M4N and YM155, specifically inhibit Survivin and Cdk4 in vitro (Figure 3) and in vivo (Figure 6), could be the major mechanisms for the observed cell arrest (Figure 2) and the decreased cell proliferation (Figure 6a and b). In addition, interference with the nuclear translocation (Figure 4a and b) and the progression through mitosis (Figure 4c–f) may be another venue for the compounds to slow down the growth of cancer cells, as it was reported that Survivin in the HCC cells is mostly nuclear,9, 10 and the nuclear location is thought to be associated with the S-phase progression.29 The observation that IMS compounds (especially IBN-9) are capable of simultaneously suppressing Survivin (and its nuclear translocation) and Cdk4, thereby leading to cell arrest and apoptosis in HCC cells in vitro and in vivo represent a novel example for suppressing HCC. Although IBN-1 and IBN-9 did not significantly alter the expression of proteins relevant in p53 signaling (Supplementary Figure S2), we cannot be certain that Survivin and Cdks are the only two major pathways affected by the two IMS compounds.
An intriguing observation made from the current study was that IBN-1 and IBN-9 with an IC50 value of around 100 μM in vitro turned out to have a reasonable in vivo efficacy (eg, 60% tumor reduction by IBN-9). In fact, similar findings had been documented in multiple tumor types. For instance, the STAT3 inhibitor NSC74859 with an in vitro IC50 value in monoculture close to 100 μM effectively inhibited the growth of breast cancer30 and HCC31 in xenograft mice. Another example was the Cdk7 inhibitor BS-181, which had an IC50 of 15–30 μM in multiple cell lines, reduced the breast tumor in mouse model by >50%.12 The enhanced anti-tumor efficacy in vivo could be indirectly mediated by the compound's effect on reducing the stromal contribution to carcinoma formation and growth, with TGF-β1 being one of the key stromal tumor-promoting components.16 It was found that HCC cells with a disrupted TGF-β1 signaling were more susceptible to NSC74859.31 A compound (Tranilast) with anti-TGF-β1 property was recently shown to be effective in inhibiting the growth of primary mammary carcinoma and the metastasis to lung.17 Similarly, IBN-1 (also called DBZIM) with an anti-TGF-β1 property in HSCs14 may have indirectly contributed to the anti-HCC effect in vivo observed in the current study. These observations cautioned us that screening and judging compounds by IC50 values generated in a monoculture system in vitro alone may be inadequate or sometimes be misleading to obtain compound candidates with satisfactory efficacy, and PK/PD attributes in vivo. We also realized that the xenograft mouse model has a relatively low predictive value in developing human therapeutics, especially for compounds that may work through the stromal cells. Hence, the use of orthotopic model, ideally using primary cells derived from human HCC, would be a more accurate method for predicting compound efficacy.
In conclusion, we have synthesized and identified two metal-free IMS compounds with anti-tumor properties in HCC xenograft mouse model. Based on this study, these compounds may inhibit the HCC growth at least through the suppression of Survivin and Cdk4, as well as through the modulation of the stromal microenvironment. However, these need to be confirmed by more thorough causative experiments in future. Nevertheless, the current study reveals interesting findings on the novel anti-tumor properties of a class of unique compounds. Further, SAR and mechanistic studies on the IMS compounds would be necessary and beneficial to prove IMSs as a unique chemical pool for generating anti-tumor compound candidates with favorable mechanisms of action.
References
Thomas MB, Zhu AX . Hepatocellular carcinoma: the need for progress. J Clin Oncol 2005;23:2892–2899.
Thorgeirsson SS, Grisham JW . Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 2002;31:339–346.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390.
Altieri DC . Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 2008;8:61–70.
Nakahara T, Takeuchi M, Kinoyama I, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res 2007;67:8014–8021.
Satoh T, Okamoto I, Miyazaki M, et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res 2009;15:3872–3880.
Rodel F, Frey B, Leitmann W, et al. Survivin antisense oligonucleotides effectively radiosensitize colorectal cancer cells in both tissue culture and murine xenograft models. Int J Radiat Oncol Biol Phys 2008;71:247–255.
Tsuruma T, Iwayama Y, Ohmura T, et al. Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med 2008;6:24.
Ito T, Shiraki K, Sugimoto K, et al. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology 2000;31:1080–1085.
Moon WS, Tarnawski AS . Nuclear translocation of survivin in hepatocellular carcinoma: a key to cancer cell growth? Hum Pathol 2003;34:1119–1126.
Malumbres M, Barbacid M . Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009;9:153–166.
Ali S, Heathcote DA, Kroll SH, et al. The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer Res 2009;69:6208–6215.
Zhao L, Zhang C, Zhuo L, et al. Imidazolium salts: a mild reducing and antioxidative reagent. J Am Chem Soc 2008;130:12586–12587.
Zhang CY, Zhang YG, Ying JY, et al. A class of synthetic imidazolium salts possesses anti-oxidative and anti-fibrotic properties in hepatic stellate cells. Free Radic Res 2009;9:1–14.
Friedman SL . Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–172.
Bhowmick NA, Neilson EG, Moses HL . Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332–337.
Chakrabarti R, Subramaniam V, Abdalla S, et al. Tranilast inhibits the growth and metastasis of mammary carcinoma. Anticancer Drugs 2009;20:334–345.
Harlow KJ, Hill AF, Welton T . Convenient and general synthesis of symmetric N,N’-disubstituted imidazolium halides. Synthesis 1996;6:697–698.
Zhang C, Zhuo L . Epigallocatechin gallate and genistein attenuate glial fibrillary acidic protein elevation induced by fibrogenic cytokines in hepatic stellate cells. Int J Mol Med 2006;18:1141–1151.
Jiang X, Wilford C, Duensing S, et al. Participation of Survivin in mitotic and apoptotic activities of normal and tumor-derived cells. J Cell Biochem 2001;83:342–354.
Yang ZF, Ngai P, Ho DW, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology 2008;47:919–928.
Ma S, Lee TK, Zheng BJ, et al. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008;27:1749–1758.
Wang J, Hou T . Drug and drug candidate building block analysis. J Chem Inf Model 2010;50:55–67.
Hindi KM, Panzner MJ, Tessier CA, et al. The medicinal applications of imidazolium carbene-metal complexes. Chem Rev 2009;109:3859–3884.
Teyssot ML, Jarrousse AS, Chevry A, et al. Toxicity of copper(I)-NHC complexes against human tumor cells: induction of cell cycle arrest, apoptosis, and DNA cleavage. Chemistry 2009;15:314–318.
Ray S, Mohan R, Singh JK, et al. Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J Am Chem Soc 2007;129:15042–15053.
Malhotra SV, Kumar V . A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorg Med Chem Lett 2010;20:581–585.
Chang CC, Heller JD, Kuo J, et al. Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc Natl Acad Sci USA 2004;101:13239–13244.
Suzuki A, Hayashida M, Ito T, et al. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activation. Oncogene 2000;19:3225–3234.
Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 2007;104:7391–7396.
Lin L, Amin R, Gallicano GI, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene 2009;28:961–972.
Acknowledgements
This work was supported by the Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gopalan, B., Ke, Z., Zhang, C. et al. Metal-free imidazolium salts inhibit the growth of hepatocellular carcinoma in a mouse model. Lab Invest 91, 744–751 (2011). https://doi.org/10.1038/labinvest.2011.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2011.4