Abstract
The development of proliferative podocytopathies has been linked to ligation of tumor necrosis factor receptor 2 (TNFR2) expressed on the renal parenchyma; however, the TNFR2-positive cells within the kidney responsible for podocyte injury are unknown. We detected de novo expression of TNFR2 on podocytes before hyperplastic injury in crescentic glomerulonephritis of mice with nephrotoxic nephritis, and in collapsing glomerulopathy of Tg26HIV/nl mice, kd/kd mice, and human beings. We further found that serum levels of soluble TNF-α and TNFR2 correlated significantly with renal injury in Tg26HIV/nl mice. Thus, we asked whether ligand binding of TNFR2 on podocytes ex vivo precipitates the characteristic proliferative and pro-inflammatory diseased podocyte phenotypes. Soluble TNF-α activated NF-κB and dose-dependently induced podocyte proliferation, marked by the expression of the podocyte G1 cyclin and NF-κB target gene, cyclin D1. Microarray gene and chemokine protein expression profiling showed a marked pro-inflammatory NF-κB signature, and activated podocytes secreting CCL2- and CCL5-induced macrophage migration in transwell assays. Neutralization of TNFR2 on podocytes with blocking antibodies abrogated NF-κB activation and the induction of cyclin D1 by TNF-α, and identified TNFR2 as the primary receptor that induced IκBα degradation, the initiating event in NF-κB activation. These results suggest that TNFR2 expressed on podocytes and its canonical NF-κB signaling may directly interpose the compound pathogenic responses by podocytes to TNF-α, in the absence of other TNFR2-positive renal cell types in proliferative podocytopathies.
Similar content being viewed by others
Main
Mounting evidence suggests that podocytes, known susceptible targets of disparate immune mediators causing proteinuria,1, 2, 3 may themselves subsequently elaborate effectors such as chemokines, cytokines, and growth factors that further amplify and shape the pattern of glomerular injury;3, 4, 5, 6, 7 however, the molecular pathways responsible for these compound diseased podocyte phenotypes and how they contribute to the development of specific podocytopathies are not well understood.8, 9 In most other non-lymphoid cell types that serve as both targets and effectors of immunological challenges, the resulting phenotypes are often induced by activated NF-κB, transcription factors of paramount importance in directing localized inflammatory responses.10, 11 This fact raises the possibility that immunological challenges that potently activate NF-κB in podocytes are candidates to both precipitate and aggravate proteinuric diseases.12
With regard to the proliferative podocytopathies, crescentic glomerulonephritis and collapsing glomerulopathy (CG),13 the tumor necrosis factor-α (TNF-α)–TNF receptor 2 (TNFR2) axis has emerged as a candidate pathway contributing to the compound proliferative and pro-inflammatory diseased podocyte phenotypes within these lesions.14, 15 The TNFR family and its ligands are major membrane proximal signaling axes that robustly activate NF-κB,11 of which TNF-α is required for the development of crescentic glomerulonephritis in several animal models.16 In addition to its well-known role in priming pro-inflammatory tissue responses,10, 11 TNF-α can also function as a potent mitogen,17, 18 and recent studies in mice with crescentic glomerulonephritis from nephrotoxic nephritis showed that, of its two receptors, TNFR1 and TNFR2, the pathogenic contribution from TNFR2 predominates.19 Chimeric mice bearing TNFR2+/+ bone marrow-derived cells and TNFR2−/− renal cells were protected from hyperplastic podocyte injury, albuminuria, and glomerulosclerosis, despite intact immunity and glomerular deposition of IgG.19 This protection did not occur in TNFR2+/+ mice lacking TNFR1,19 also supporting observations in kidney explants that apoptotic vs proliferative responses of the renal parenchyma to TNF-α segregate with renal expressed TNFR1 vs renal expressed TNFR2, respectively.20 Moreover, the characteristic recruitment of inflammatory mononuclear phagocytes that intimately admix with diseased podocytes,21, 22 aggravating glomerular injury and proteinuria,23 was absent in the chimeras. This suggests that TNFR2 signaling by resident glomerular cells also contributes to detrimental chemoattraction of leukocytes.
The premise that this pathogenic role of the TNF-α–TNFR2 axis in crescentic glomerulonephritis may extend to CG is now well grounded. Multiple early reports, including observations of their morphological patterns co-existing in nephrotoxic nephritis,24 suggested that crescentic glomerulonephritis and CG develop along closely linked pathomechanisms (reviewed in ref. 8). Recently, careful cell lineage-tracing studies extended and solidified this premise.25, 26 Nearly identical admixtures of hyperplastic podocytes (visceral and parietal), parietal ‘transitional’ podocyte progenitors, and glomerular epithelial stem cells, along with recruited mononuclear phagocytes, populate and form the extracapillary proliferative lesions in crescentic glomerulonephritis and CG.25, 26 Intriguingly, CD44, a known NF-κB target gene induced by TNF-α that coordinately increases the affinity of CD44 for its ligands,27 was shown to be a glomerular epithelial cell injury marker in these studies.26
These seminal observations leave unresolved whether activation of TNFR2 on podocytes vs TNFR2 on other renal cell types contributes to podocyte injury in the proliferative podocytopathies. Nevertheless, podocytes have been documented to pathogenically respond to TNF-α. Podocytes challenged briefly with TNF-α ex vivo undergo cytoskeletal reorganization,28 shed and downregulate nephrin,29, 30 produce reactive oxygen species and inducible nitric oxide synthase (iNOS),31, 32 upregulate TNFR233 and Toll-like receptor 2 (TLR2) expression,34 and activate p38MAPK signaling,35 a pathway downstream of TNFR2.15 Unlike TNFR1, which is basally expressed by most cells at steady state, including podocytes,36 TNFR2 expression is largely restricted to leukocytes, and in normal animal and human kidneys, podocytes do not express detectable TNFR2.14, 15 However, recent examination of TNFR2 expression in glomeruli from patients with acutely rejecting renal allografts37 and IgA nephropathy33 showed that TNFR2 can be induced on podocytes. This suggests that TNFR2 may serve to directly interpose podocyte injury to TNF-α during inflammatory renal states in vivo, even in the absence of other TNFR2-positive renal cell types. Thus, we ask here whether ligand binding of TNFR2 on podocytes ex vivo precipitates the characteristic proliferative and pro-inflammatory diseased podocyte phenotypes.
MATERIALS AND METHODS
Mice
All animal studies were approved by the respective Institutional Animal Care and Use Committees. The C57Bl/6 (B6) nephrotoxic nephritis model of crescentic glomerulonephritis,38 the heterozygous FVB/N Tg26 human immunodeficiency virus (HIV) (Tg26HIV/nl) transgenic mouse model of CG,39 and the B6 kd/kd model of CG40 have been characterized and described in detail. Kidneys from nephrotoxic nephritis mice were collected 3 days after injection of antibody, before the onset of significant hyperplastic glomerular injury. Serum, urine, and kidneys were collected from Tg26HIV/nl mice ranging between 2 and 4 months of age, and urinalysis and serum chemistries were performed as described previously.41 Mice were maintained under specific pathogen-free conditions.
Microscopy
Immunohistochemical detection of TNFR2 was performed on kidney sections from nephrotoxic nephritis mice, Tg26HIV/nl mice, kd/kd mice, normal wild-type FVB/N and B6 mice, and on archival human kidney biopsies diagnosed with CG (n=10), primary focal segmental glomerulosclerosis (n=10), and normal kidney on post-transplant protocol biopsy (n=2). All studies on human tissues were Institutional Review Board approved. Paraffin sections (3-μm thick) underwent low pH antigen retrieval (Dako, Carpinteria, CA, USA), followed by staining for TNFR2 (1:100; rabbit polyclonal; GeneTex, Irvine, CA, USA) using 3,3′-diaminobenzidine staining kits from Vector Laboratories (Burlingame, CA, USA) as per the manufacturer's protocols. Immunofluorescence detection of TNFR2 on differentiated podocytes on glass coverslips was performed after challenge with TNF-α (2.5 ng/ml) for 24 h to enhance TNFR2 expression. Podocytes were fixed with 60% acetone/3.7% formaldehyde for 20 min at −20 °C and stained for TNFR2 (1:100; rabbit polyclonal; GeneTex) using polyclonal FITC-labeled swine anti-rabbit antibody (1:20; Dako, Carpinteria, CA, USA) for secondary detection. Quantitative histopathology on periodic acid-Schiff-stained slides of kidneys from Tg26HIV/nl mice was performed as described previously.40
Cell Culture
The conditionally immortalized podocyte cell culture system, originally developed by Mundel and co-workers42 and used previously by us for culturing normal43 or HIV-expressing Tg26HIV/nl podocytes,44 was employed for studies on podocytes ex vivo. The protocol for developing undifferentiated or differentiated podocytes in cell culture has been described in detail.42 The proliferative response of podocytes to soluble recombinant mouse TNF-α (R&D Systems, Minneapolis, MN, USA) or vehicle (phosphate-buffered saline, pH 7.4) was determined by CellTiter 96 Non-Radioactive Cell Proliferation assays (Promega, Madison, WI, USA). Triplicate wells of 2000 podocytes per well, either growth restricted (37 °C with no interferon-γ for 24 h) or differentiated (37 °C with no interferon-γ for 14 days), were treated for 6 days with twofold dilutions of TNF-α (10 ng/ml–5 pg/ml) or vehicle under continuing growth-restricted conditions before CellTiter assays. Additional wells of differentiated podocytes were treated with TNF-α (2.5 ng/ml) or vehicle for 6 days, fixed with 60% acetone/3.7% formaldehyde at −20 °C for 20 min, and stained with 0.4% Trypan blue to visualize cell density and morphology by light microscopy. Changes in protein expression by differentiated podocytes following challenge with or washout of TNF-α (2.5 ng/ml) or vehicle were evaluated by western blot. Fifty micrograms of protein lysates underwent denaturing polyacrylamide gel electrophoresis and immunoblotting for cyclin D1 (clone SP4; LabVision, Fremont, CA, USA), TNFR2 (rabbit polyclonal; GeneTex), and tubulin-β (rabbit polyclonal; LabVision), using horse radish peroxidase-conjugated antibodies for secondary detection. Western blot band intensity by chemiluminescence (Supersignal West Femto Maximum Sensitivity Substrate; Thermo Fisher Scientific, Milwaukee, WI, USA) was quantified on a ChemiDoc EQ system (BioRad Laboratories, Hercules, CA, USA) using QuantityOne software (BioRad Laboratories). All experiments were repeated three separate times for significance.
Evaluation of both baseline mRNA levels and parallel changes in mRNA expression with TNF-α treatment by quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed by the New York University Cancer Institute Genomics Facility with primers designed by Primer3 (software available at http://www-genome.wi.mit.edu/genome_software/other/primer3.html). Primer sequences for target genes were as follows: cyclin D1—forward, CACAACGCACTTTCTTTCCA, reverse, TCCAGAAGGGCTTTCAATCTG; iNOS—forward, AGCTGAACTTGAGCGAGGAG, reverse, GGAAAAGACTGCACCGAAGA; and WT1—forward ACCATCTGAAGACCCACACC, reverse, ACAACTGTGCCACCGACAG. Primer sequences for internal controls were as follows: β-2-microglobulin—forward, CTGACCGGCCTGTATGCTAT, reverse, TATGTTCGGCTTCCCATTCT; glyceraldehyde-3-phosphate dehydrogenase—forward, GGCATTGCTCTCAATGACAA, reverse, CCCTGTTGCTGTAGCCGTAT; and hypoxanthine phosphoribosyl-transferase I—forward, TGTTGTTGGATATGCCCTTG, reverse, GGCTTTGTATTTGGGCTTTTCC. For each triplicated quantitative RT-PCR reaction, 10 ng of cDNA generated by iScript cDNA Synthesis reagents (BioRad Laboratories) from whole podocyte RNA (collected by TRI Reagent, Molecular Research Center, Cincinnati, OH, USA) underwent amplification and PCR product detection on an ABI 7900 SDS cycler (Applied Biosystems, Foster City, CA, USA) using Power SYBR Green PCR Master Mix reagents (Applied Biosystems). Determination by the 2−ΔΔCt method of the fold change in the expression of each target gene relative to three geometrically averaged internal reference genes, previously shown to increase the accuracy of quantitative RT-PCR,45 was performed as described previously.46 Data are presented as fold change in expression relative to baseline expression of each gene.
NF-κB Assays
Normal podocytes and HIV-expressing Tg26HIV/nl podocytes,44 cultured under the conditions described above, were examined with a functional assay for NF-κB activation using a standard reporter assay in 24-well plates (2.5 × 104 cells per well) as described previously.44 This assay involves transient transfection of a plasmid (pNF-κB SEAP (secreted alkaline phosphatase); Clontech, Mountain View, CA, USA) expressing the reporter gene SEAP under the control of an NF-κB-dependent promoter. At 24 h after transfection, media were removed and replaced with media containing either vehicle (phosphate-buffered saline), TNF-α (20 ng/ml), lipopolysaccharide (LPS) (O55:B5, 1 ng/ml; Sigma, St Louis, MO, USA), or combinations containing rat anti-mouse TNF-α-neutralizing IgG (20 μg/ml, clone MP6-XT22; eBiosciences, San Diego, CA, USA) or TNF-α-neutralizing soluble (s)TNFR1 Fc-fusion protein (5 μg/ml; R&D Systems). After 24 h, SEAP levels in conditioned media were determined by chemiluminescence (BD Biosciences, San Jose, CA, USA) as described previously.44 Reporter assay results were confirmed by western blotting or RT-PCR of native genes regulated by NF-κB (TNFR2, iNOS, or cyclin D1).
Neutralization of TNFR1 or TNFR2 on podocytes was achieved with monoclonal hamster anti-mouse IgG blocking TNFR1 (clone 55R-170; BioLegend, San Diego, CA, USA) or blocking TNFR2 (clone TR75-54.7; BioLegend), originally developed by Sheehan et al.47 Nonspecific monoclonal hamster IgG (clone HTK888; BioLegend) was used as control. We confirmed (data not shown) that these blocking antibodies do not have agonistic activity when bound to the receptors, as shown previously47 and stated by the manufacturer. Differentiated podocytes were challenged with vehicle (phosphate-buffered saline) or TNF-α for 24 h in the absence or presence of 2-log dilutions (1000–0.01 ng/ml) of each antibody before collection of media or RNA for SEAP measurements or quantitative RT-PCR analyses, respectively. All studies were performed at least three times in triplicate for statistical analysis.
Measurement of IκBα degradation in podocytes, evaluated by western blotting as described previously,44 was also carried out in parallel with SEAP measurements during blocking of TNFRs with neutralizing antibodies. As above, podocytes were pretreated with neutralizing antibodies (1000 ng/ml) followed by stimulation with TNF-α (20 ng/ml) for 24 h. Owing to the normally high rate of IκBα turnover, cells were re-stimulated with TNF-α for 30 min before lysis. The western blotting studies were repeated three times for statistical analysis.
Gene Microarray Profiling
RNA was extracted from quiescent, differentiated normal podocytes before challenge with TNF-α, or after a 48 h treatment with TNF-α (2.5 ng/ml), on three separate occasions. RNA (100 μg) from each extraction was hybridized on Affymetrix Mouse 430A 2.0 genes chips (New York University Cancer Institute Core Genomics Facility, New York, NY, USA). Normalization, data filtering, and identification of statistically significant differential mRNA abundance, defined as twofold or greater on average between quiescent and TNF-α-activated podocytes, were performed as described previously.48
Migration and Cytokine Assays
Migration assays were performed on transwells (24-well format, 5.0-μm uncoated polycarbonate) using the murine macrophage/monocyte cell line RAW264.7 (American Type Culture Collection, Manassas, VA, USA; clone TIB-71). Chemoattractants were 3-day conditioned media from normal or Tg26HIV/nl podocytes, stimulated, and unstimulated with TNF-α. Five hundred and fifty microliters of conditioned media, 0.2 μm filtered before use, were applied to the lower transwell chamber. RAW264.7 cells were washed three times in phosphate-buffered saline, suspended in media containing 0.5% fetal bovine serum, and applied to the upper chamber (105 cells in 100 μl). Chambers were incubated for 4 h at 37 °C. Cells remaining in upper chambers were gently removed, and migrated cells were fixed and stained with a Hema 3 staining kit (Thermo Fisher Scientific) as per the manufacturer's protocol. Stained cells were manually counted in six randomly selected fields, and each experiment was performed three times.
Chemokines from the conditioned media were quantified using a bead-based, antigen–antibody multi-analyte profiling assay (LINCOplex, Millipore, Billerica, MA, USA) analyzed on a Luminex LabMAP (Luminex Corp., Austin TX, USA). Mouse serum TNF-α levels were quantified using a Fluorkine multi-analyte profiling assay (R&D Systems) analyzed on a Luminex LabMAP. Mouse serum sTNFR levels were quantified using colorimetric enzyme-linked immunosorbent assays (Quantikine; R&D Systems). All samples were tested in duplicate and duplicates were averaged before statistical analysis.
Gene Silencing by RNAi
TNFR2 expression was reduced in podocytes by RNAi using commercially available, pre-made RNAis (ON-TARGETplus SMARTpool L-043973; Dharmacon). A non-targeting RNAi was used as a control (ON-TARGETplus D-001810; Dharmacon). The RNAis were transfected into cells as recommended by the manufacturer and the extent of knockdown was determined by western blotting. RNAi-transfected cells were used for experiments, including the SEAP reporter assay for functional NF-κB activation and quantification of cell proliferation as described above. For NF-κB reporter assays, the reporter plasmid was co-transfected with the RNAi. Each experiment was performed three times in duplicate.
Statistics
Unless otherwise stated, numerical and graphical data are expressed as mean±standard deviation. Comparisons were made using the two-tailed t-test. Correlations between TNF-α and sTNFR2 and between sTNFR2 and renal indices of CG were evaluated by calculating Pearson's correlation coefficients (R-values) and through simple linear regression. Significance was accepted at the 0.05 level of probability.
RESULTS
TNFR2 Expression by Podocytes in Proliferative Podocytopathies
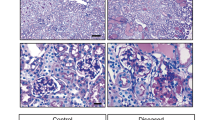
TNFR2 is normally expressed by several leukocytes subsets; however, other cell types including renal epithelial and endothelial cells can be induced to express TNFR2 by TNF-α itself, interleukin-1 family cytokines, and several other pro-inflammatory mediators.14, 15, 33, 49 As the extracellular domain of membrane TNFR2 is rapidly cleaved and shed,50, 51 we employed an antibody reactive to its intracellular domain to detect TNFR2 in situ in the crescentic glomerulonephritis of B6 mice with nephrotoxic nephritis,38 and in the CG of Tg26HIV/nl, one of the best studied models of CG,39 B6 kd/kd mice, a non-HIV model of CG,40 and human biopsies. Immunohistochemical staining showed a distinct pattern of de novo expression of TNFR2 by podocytes in crescentic glomerulonephritis and CG. Podocytes in normal FVB/N mice (Figure 1a) and normal B6 mice (not shown) did not have detectable TNFR2. In contrast, podocytes in nephrotoxic nephritis kidneys (Figure 1b), in Tg26HIV/nl kidneys (Figure 1c and d), and in kd/kd kidneys (Figure 1e and f) clearly expressed TNFR2 before the development of hyperplastic injury in glomeruli. In full coronal sections, adjacent glomeruli at varying stages of hyperplastic injury and fibrosis were identifiable and highlighted the differential expression of TNFR2 (Figure 1d and f).
Expression of tumor necrosis factor receptor 2 (TNFR2) by podocytes in proliferative podocytopathies. (a) Lack of TNFR2 expression by podocytes in a normal FVB/N kidney. (b) TNFR2 expression by podocytes in the glomerulus from a nephrotoxic nephritis kidney before significant hyperplastic injury. (c) TNFR2 expression by podocytes in a diseased Tg26HIV/nl kidney in a glomerulus with little to no hyperplastic injury. (d) Differential TNFR2 expression by podocytes in a diseased Tg26HIV/nl kidney in adjacent glomeruli, one (long arrow) with little to no hyperplasia, and the other (short arrow) with marked hyperplasia and fibrosis. (e) TNFR2 expression by podocytes in a diseased kd/kd kidney in a glomerulus with little to no hyperplastic injury. (f) Similar to (d), differential TNFR2 expression by podocytes in a diseased kd/kd kidney in adjacent glomeruli, one (long arrow) with little to no hyperplasia, and the other (short arrow) with marked hyperplasia. (g) Lack of TNFR2 expression by podocytes in a human biopsy diagnosed with primary focal segmental glomerulosclerosis. (h) TNFR2 expression by podocytes in a human biopsy diagnosed with CG in a glomerulus with little to no hyperplasic injury. (i) Higher power view of podocytes (arrows) in (h). Magnifications: × 400 in (a, b, c, e, and i); × 200 in (d, f, g, and h).
Contrary to recent reports suggesting shared pathomechanisms in CG and crescentic glomerulonephritis,8, 25, 26 CG has been classically described as a variant of primary focal segmental glomerulosclerosis, a lesion characterized by podocyte loss, not hyperplastic injury.52 We therefore examined TNFR2 expression in 10 human biopsies with CG, in 10 human biopsies with primary focal segmental glomerulosclerosis, and in two human protocol transplant biopsies showing normal kidney. As described by others,33 TNFR2 was not detected on podocytes in normal human kidneys, nor was it detected on podocytes in primary focal segmental glomerulosclerosis (Figure 1g). In CG, however, TNFR2 was detected on human podocytes in glomeruli without the evidence of significant hyperplastic injury and capillary collapse at the time of biopsy (Figure 1h and i). These well-preserved glomeruli were infrequent, being present in only two of the 10 archival cases studied, indicative of the state of parenchymal injury at clinical presentation of CG. Taken together, this pattern of de novo expression of TNFR2 by podocytes in animals and human beings before significant hyperplastic glomerular injury suggests that its engagement by TNF-α may contribute to directly precipitating the proliferative podocytopathies.
TNF-α and TNFR2 in CG
The development of crescentic glomerulonephritis is well studied for its dependency on TNF-α,16 an innate immune and T-helper type 1 (Th1) effector cytokine; however, this has not been explored in CG. Nevertheless, reports of CG predominate with patients afflicted by pre-existing Th1-polarizing disorders originating outside the kidney, the most prevalent being HIV infection, suggesting a causal link similar to crescentic glomerulonephritis.8, 52 Thus, we studied this clinical correlate in detail in Tg26HIV/nl mice, which display abnormally elevated serum TNF-α53 from the co-opting of TNF-α expression control by HIV-1 gene products.54 Serum levels of sTNF-α and its sTNFRs (shed from membranes to delimit the activity of TNF-α50, 51), and the extent of renal dysfunction and injury was evaluated in diseased Tg26HIV/nl mice and non-transgenic littermates. Compared with non-transgenics, diseased Tg26HIV/nl mice had significantly higher levels of serum TNF-α, sTNFR1, and, most prominently, sTNFR2 (Figure 2a and b), consistent with the upregulation, cleavage, and shedding of TNFR2 during inflammation mediated by TNF-α.50, 51 Serum levels of TNF-α correlated significantly with sTNFR2 (Figure 2c). As sTNFR2 is a more robust and dynamic surrogate of TNF-α activity on target tissues than sTNFR1,50, 51 sTNFR2 was compared with indices of CG in a large cohort of Tg26HIV/nl mice exhibiting a wide range of renal disease (Table 1). This analysis showed that the levels of sTNFR2 correlated significantly with the degree of podocyte proliferation, podocyte injury (by desmin expression), global glomerular capillary collapse and sclerosis, tubulointerstitial microcysts and inflammation, proteinuria, and blood urea nitrogen, but not the milder histopathological parameters (segmental glomerulosclerosis and collapse) or the dyslipidemia of nephrotic syndrome (serum triglycerides and cholesterol levels). The strength of the association data was determined with Pearson's correlation coefficients, and were found to be good (Table 1; R-values between 0.5 and 0.8) for proteinuria, blood urea nitrogen, global glomerulosclerosis, podocyte injury, and tubulointerstitial inflammation, whereas the association data were weaker (R-values less than 0.5), but significant for the two key features of CG, global glomerular collapse (R=0.464) and podocyte hyperplasia (R=0.347), as well as tubular microcysts (R=0.383). In composite, these results showed a significant and overall good correlation between TNF-α expression and activity and the development of renal disease in Tg26HIV/nl mice. Taken together with the de novo expression of TNFR2 on podocytes, we hypothesized that TNF-α–TNFR2 interaction induces the characteristic proliferative and pro-inflammatory diseased podocyte phenotypes ex vivo.
Levels of soluble tumor necrosis factor-α (TNF-α), soluble TNFR1 (sTNFR1), and sTNFR2 in the serum of Tg26HIV/nl mice. Concentrations of (a) soluble TNF-α and (b) sTNFR1 and sTNFR2 in the serum of diseased Tg26HIV/nl mice and normal non-transgenic littermates. (c) Linear regression analysis and scatter plot showing a significant correlation between serum levels of soluble TNF-α and sTNFR2 levels in normal and Tg26HIV/nl mice with a range of severity of renal disease.
Proliferative Phenotype of Podocytes to TNF-α
TNF-α is a cytokine promoter of proliferation and transformation for many epithelial and other cell types, particularly during chronic immune activation and inflammation.17, 18 To address this possibility for podocytes ex vivo, we employed the podocyte cell culture system developed by Mundel and co-workers42 and utilized previously by us43 to study their phenotypic response to prolonged challenges with sTNF-α. Differentiated podocytes exhibited robust, dose-dependent proliferation to sTNF-α quantified using a standard proliferation assay (MTS assay; Figure 3a). These cultures had abundant mitotic figures and foci formation by day 6 of TNFα treatment (Figure 3b and c). TNF-α similarly induced proliferation of undifferentiated podocytes (Figure 3a, growth restricted). Notably, however, unlike undifferentiated podocytes growing under permissive conditions (dependent on a temperature-sensitive SV40 T antigen, a mechanism of viral transformation), which downregulate expression of WT1,42 differentiated podocytes induced to proliferate via sTNF-α retained gene expression of WT1, as previously observed by Takano et al.32 Quantitative RT-PCR analysis of podocytes proliferating with TNF-α vs podocytes proliferating via SV40 T showed that WT1 expression was increased (+1.16±0.03-fold) vs decreased (−22.17±0.50-fold), respectively, when compared with quiescent, differentiated podocytes without TNF-α stimulation. TNF-α stimulation of differentiated podocytes did not reactivate the temperature-sensitive SV40 T antigen (Supplementary Figure 1).
Proliferative response of podocytes to soluble tumor necrosis factor-α (TNF-α). (a) Cell proliferation assay showing dose-dependent proliferation of podocytes, either differentiated for 14 days or growth restricted for 1 day, to a 6-day challenge with vehicle or twofold increasing doses of soluble TNF-α. (b) Representative monolayer of differentiated podocytes challenged with vehicle for 6 days, and then fixed and stained with Trypan blue. (c) Representative changes in the density and morphology of differentiated podocytes challenged with TNF-α (2.5 ng/ml) for 6 days, and then fixed and stained with Trypan blue. Several mitotic figures (arrows) and foci (above asterisks) are present. Magnification: × 100 in (b) and (c). (d) Representative western blot analyses of the changes in cyclin D1 protein expression by differentiated podocytes lysed after a 6-day challenge with TNF-α (2.5 ng/ml; lane 2) or vehicle (lane 4), or lysed at day 12 after a 6-day challenge with and then a 6-day washout of TNF-α (2.5 ng/ml; lane 3) or vehicle (lane 5). Lysates from differentiated podocytes before challenge with TNF-α at day 0 (lane 1) are included. The induction of TNFR2 by TNF-α is also shown as a positive control. (e) Fold change in the protein expression of cyclin D1 relative to tubulin-β under the conditions of (d).
Pathogenic cell-cycle engagement of quiescent (G0) podocytes in the proliferative podocytopathies is driven by the expression of the G1 cyclin, cyclin D1,43, 55 a known target gene of TNF-α,17, 18 and of NF-κB.56, 57 Thus, we selected cyclin D1 as a proliferation marker of TNF-α–TNFR2 signaling by podocytes and studied its gene expression by quantitative RT-PCR before, during, and after (ie, washout) challenge with sTNF-α. iNOS, a previously-identified NF-κB target gene of TNF-α in podocytes,32 was used as a positive control. As with iNOS, cyclin D1 was upregulated during challenge with TNF-α and returned to near baseline following washout of TNF-α, with baseline defined as the expression before challenge with TNF-α (Table 2). This was also evident in the pattern of protein expression of cyclin D1 (Figure 3d and e). Taken together, these results indicate that TNF-α can serve as a mitogenic cytokine for podocytes, even when the podocytes were in a differentiated, quiescent state.
Pro-inflammatory NF-κB Activation in Podocytes by TNF-α
Although unknown for podocytes, signaling via the TNF-α–TNFR2 axis activates the pro-inflammatory transcription factor NF-κB in other cell types.11 We have previously shown NF-κB is persistently activated in HIV-expressing Tg26HIV/nl podocytes,44 resulting in a higher baseline activation level compared with normal podocytes. We therefore studied NF-κB activation in both normal and Tg26HIV/nl podocytes following challenges with TNF-α ex vivo. As expected, stimulation of both normal and Tg26HIV/nl podocytes with sTNF-α induced or further increased NF-κB activation, respectively (Figure 4). Blocking the sTNF-α with neutralizing antibody or chimeric TNFR1 abrogated this NF-κB activation, showing the specificity of the recombinant TNF-α preparation and its lack of contamination by endotoxin, as control LPS-induced NF-κB activation was not countered by blocking TNF-α (Figure 4).
Functional activation of nuclear factor-κB (NF-κB) in podocytes by soluble tumor necrosis factor-α (TNF-α). (a) Normal and (b) Tg26HIV/nl podocytes were transfected with an NF-κB-dependent secreted alkaline phosphatase (SEAP) reporter plasmid to functionally assay NF-κB transcriptional activation. Stimulation with soluble TNF-α or lipopolysaccharide (LPS) strongly induced NF-κB activity in both normal and Tg26HIV/nl podocytes. Neutralization of TNF-α with either antibody or soluble chimeric TNF receptor1 (TNFR1) eliminated NF-κB activation by TNF-α, but not by LPS (**P<0.005; ***P<0.001).
TNF-α is well characterized for inducing NF-κB-mediated pro-inflammatory priming of tissues, leading to the recruitment of leukocytes.11 To capture the broad repertoire of gene responses whereby podocytes may interpose this effect of TNF-α in the proliferative podocytopathies, RNA from podocytes challenged with TNF-α were profiled on Affymetrix Mouse 430A 2.0 microarray genes chips. Compared with quiescent podocytes, 183 annotated genes were induced twofold or greater in podocytes activated by TNF-α (Supplementary Table 1). Not surprisingly, the NF-κB cell-cycle target gene, cyclin D1, and a large host of downstream mitotic genes (eg, cyclin E1, Ki-67, PCNA) involved in cell-cycle progression, DNA replication, cytokinesis, and suppression of apoptosis (eg, survivin) of podocytes were induced by TNF-α. The expression of pro-inflammatory genes was also pronounced and included TNFR2, multiple chemokines, cell adhesion and receptor proteins (including TLR2 and CD44, as described recently26, 34), antigen-presenting molecules, acute phase reactants, and transcription factors and regulators of the NF-κB family itself (Table 3), but not TNF-α. Cross-referencing with known target genes of activated NF-κB showed that the vast majority of these pro-inflammatory genes fall into this category.
As recruited inflammatory mononuclear phagocytes intimately admix with diseased podocytes in CG8 and crescentic glomerulonephritis,21, 22 we sought to validate the known functionality of the NF-κB-induced macrophage chemokines, CCL2 (MCP-1) and CCL5 (RANTES),58 present in this NF-κB target gene signature. Conditioned media from normal and Tg26HIV/nl podocytes induced macrophage migration, and with TNF-α stimulation, this chemoattractive effect was significantly increased in both cell types (Figure 5). The conditioned media contained readily detectable CCL2 and CCL5 protein (Table 4) each secreted two- to threefold greater by Tg26HIV/nl podocytes, consistent with their higher degree of NF-κB activation (Figure 4). In comparison, other candidate NF-κB-dependent chemokines were also readily detected, but did not show the same differential secretion by Tg26HIV/nl podocytes (Table 4), suggesting that HIV-expressing podocytes may harbor specific functional consequences on leukocyte recruitment. Similar to the mRNA expression profiling results above, TNF-α was not detectable in conditioned media from normal or Tg26HIV/nl podocytes, suggesting that NF-κB activation or sTNF-α treatment did not induce new TNF-α production. Taken together, these results suggest that podocytes can directly serve as effectors of the pro-inflammatory and chemoattractive effects of TNF-α in diseased glomeruli.
Chemoattraction of macrophages to podocytes-derived chemokines. Migration of monocytic cells (murine cell line RAW264.7) was used as a functional assay for chemokine secretion by podocytes. Unconditioned media (media) and conditioned media from normal and heterozygous FVB/N Tg26 human immunodeficiency virus (Tg26HIV/nl) podocytes, either untreated or treated with tumor necrosis factor-α (TNF-α), were tested for the ability to chemoattractant macrophages in standard transwell migration assays, quantified as the number of migrated macrophages per high powered field. The identification and concentration of chemokines in the media was determined using a multi-analyte profiling assay (see Table 4). **P<0.01 compared with respective conditioned media without TNF-α stimulation.
Neutralization of TNFR2 on Podocytes
As TNFR2 is expressed by podocytes before hyperplastic injury in proliferative podocytopathies, we neutralized TNFR2 on podocytes to determine whether its engagement is required for the activation of NF-κB and the induction of cyclin D1. Application of the TNFR2-blocking antibody, TR75-54.7, originally developed by Sheehan et al,47 30 min before challenge with TNF-α, dose-dependently abrogated the functional activation of NF-κB (Figure 6a) at concentrations shown previously to neutralize TNFR2,47 whereas an isotype-matched control antibody had no effect. In contrast, the TNFR1-blocking antibody, 55R-170, also developed by Sheehan et al,47 did not abrogate functional activation of NF-κB at concentrations shown previously to neutralize TNFR1. This inability of TNF-α to activate NF-κB with neutralization of TNFR2 paralleled the loss of induction by TNF-α of the NF-κB target genes, cyclin D1 (Figure 6b) and iNOS (Figure 6c), the latter studied as a known positive control.
Neutralization of tumor necrosis factor receptor 2 (TNFR2) on podocytes and responses to TNF-α. (a) Functional nuclear factor-κB (NF-κB) activation was assessed in transient transfection of an NF-κB-dependent secreted alkaline phosphatase (SEAP) reporter. After transfection, normal podocytes were treated 30 min before a 24 h challenge with TNF-α with blocking antibodies for TNFR2 (clone TR75-54.7), TNFR1 (clone 55R-170), or an isotype-matched control immunoglobulin G (*P<0.05; **P<0.02 compared with TNF-α treated). In parallel experiments on normal, differentiated podocytes, the fold change in gene expression of (b) cyclin D1 and (c) inducible nitric oxide synthase (iNOS) induced by TNF-α during neutralization of TNFR1 or TNFR2 was compared by quantitative reverse transcription-polymerase chain reaction (RT-PCR) to the level of gene expression in podocytes before challenge with TNF-α.
We showed previously that degradation of IκBα, the key cytoplasmic repressor of canonical NF-κB activation, is associated with the NF-κB-dependent proliferative responses of podocytes in HIV-associated CG.44 Proteasomal degradation of IκBα is the key initiating event following membrane receptor engagement that induces the NF-κB activation cascade. Thus, we studied the degradation of IκBα in podocytes following challenge with TNF-α using similar receptor neutralization strategies as in Figure 6. NF-κB activation was significantly reduced with neutralization of TNFR2, not TNFR1, and their combined neutralization did not significantly differ from neutralization of TNFR2 alone (Figure 7a). Parallel examination of total IκBα protein by western blotting showed that IκBα degradation was significant only through the engagement of TNFR2, with minimal degradation mediated by TNFR1 (Figure 7b). Taken together, these studies mechanistically link selective ligation of TNFR2 with the proximal signaling events and functional gene expression from TNF-α-induced activation of NF-κB in podocytes.
Tumor necrosis factor (TNF) receptor specificity for nuclear factor-κB (NF-κB) activation. (a) Similar to Figure 6, functional NF-κB activity in podocytes following challenge with TNF-α using blocking antibodies was assessed using the secreted alkaline phosphatase (SEAP) reporter assay. (b) Western blotting for IκBα degradation in podocytes following TNF-α induction in the same experiments presented in (a). Shown is a representative western blot for IκBα that was re-probed for α-tubulin, a control protein, and a graph of the quantification of all gels normalized to α-tubulin expression. Lanes 1–6 correspond to the treatments shown below the bars on the graph (*P<0.05 compared with TNF-α treated).
To confirm the receptor neutralization studies, similar experiments for NF-κB activation and proliferation were conducted in which TNFR2 expression was reduced by RNAi-mediated gene silencing. Using RNAi, expression of TNFR2 was reduced by 64% compared with mock-transfected cells (Figure 8a). Upon stimulation with TNF-α, the RNAi knockdown cells showed significant reductions in both proliferation (68% reduction; Figure 8b) and NF-κB activation (69% reduction; Figure 8c). The reductions in both proliferation and NF-κB activation are consistent to the level of reduction in TNFR2 expression and support their mechanistic link to TNFR2 function in podocytes.
Effect of tumor necrosis factor receptor 2 (TNFR2) gene silencing on proliferation and nuclear factor-κB (NF-κB) activation. Podocytes were mock transfected (no RNA interference (RNAi)) or transfected with a non-target control or TNFR2-specific RNAi followed by treatment with TNF-α. (a) Reduction in the expression of TNFR2 by RNAi was evaluated by western blotting; a representative gel is shown and quantification indicated a reduction of 64% by TNFR2 (‘R2’) RNAi compared with mock-transfected cells (‘−’). Control (‘C’) RNAi showed an 8% reduction compared with mock-transfected cells. Expression of TNFR1 was unchanged; tubulin was used as a loading control. (b) Effect on cell proliferation with each RNAi was tested in experiments similar to Figure 3. Graphs are a composite of three experiments that resulted in an average reduction of 68% (*P≤0.05 compared with control treated with TNF-α). (c) Effect on NF-κB activation with each RNAi was tested in experiments similar to Figure 4. Graphs are a composite of three experiments that resulted in an average reduction of 69% (*P≤0.05 compared with control treated with TNF-α).
DISCUSSION
The immune induction of compound diseased podocyte phenotypes, in which podocytes are both targets and effectors of inflammatory challenges, is increasingly recognized as an important factor in the glomerular injury of several podocytopathies.3, 4, 5, 6, 7, 8, 9 We show here that TNFR2 expressed by podocytes can directly interpose the proliferative (target) and pro-inflammatory and chemoattractant (effector) phenotypes—all characteristic of diseased podocytes in the proliferative podocytopathies—induced by TNF-α. This extends the seminal observations by Vielhauer et al19 of the requirement for renal expressed TNFR2 to precipitate disease, and suggests that the capacity of the TNF-α–TNFR2 axis to injure podocytes may not necessitate other TNFR2-positive renal cell types. These results do not, however, exclude possible contributions from other TNFR2-positive cell types or pro-inflammatory mediators to podocyte injury in the proliferative podocytopathies in vivo, a question that will require selective deletion of TNFR2 from podocytes, but not from other renal and non-renal cell types to address.
The exact mechanisms for the de novo expression of TNFR2 by podocytes in the proliferative podocytopathies and in other inflammatory glomerular diseases33, 37 remain to be determined. TNFR2 can be upregulated by extrinsic factors such as pro-inflammatory cytokines (eg, by TNF-α as shown here), growth factors, and microbial products.50, 51 In support of this possibility, CG is often diagnosed in patients afflicted by extra-renal Th1-polarizing disorders,52 suggesting that these extrinsic mechanisms may be operative for podocytes. Alternatively, an intrinsic defect of podocytes may lead to the expression of TNFR2, as was shown by its induction via hypoxia-inducible factors in the podocyte-restricted von Hippel–Lindau-null model of crescentic glomerulonephritis4 or by the proposed mitochondrial-hypoxia-inducible factor pathway in the B6 kd/kd model of CG.8 In these latter instances, TNFR2-positive podocytes may become quite susceptible to otherwise ‘clinically silent’ levels of TNF-α. This was shown when CG in B6 kd/kd mice was eliminated when the mice were housed in a germ-free environment, indicating that the ‘benign’ specific pathogen-free Th1 environment, which has no effect on normal mice, can itself precipitate CG in B6 kd/kd mice.59 In any respect, contributions from synergistic pathogenic cofactors, such as underlying genetic predispositions in patients susceptible to CG,52 likely determines the pattern of glomerular injury following TNFR2 ligation on podocytes.
The Tg26HIV/nl model of CG exemplifies this multi-factorial pathogenesis associated with the TNF-α–TNFR2 axis in glomerular injury. The development of renal disease in Tg26HIV/nl mice requires both the FVB/N genetic background60 and expression of the HIV transgene.61 Within the FVB/N background, however, neutralization of TNF-α in Tg26HIV/nl mice has been shown to silence HIV transgene expression and its associated pathologies,53 showing the sensitivity of the HIV promoter to NF-κB activated by TNF-α.62 We show here an overall good correlation between TNF-α, TNFR2, and the development of renal disease in Tg26HIV/nl mice; thus, the TNF-α–TNFR2 axis in Tg26HIV/nl mice may serve not only to precipitate disease phenotypes, but also to upregulate the expression of HIV gene products known to further exacerbate tissue injury and the activation of NF-κB itself.63 This would be expected to create pathogenic feedforward NF-κB loops in target cells such as podocytes (Figure 9). Indeed, the previously described HIV-expressing podocytes from Tg26HIV/nl mice utilized here show persistent activation of NF-κB, and suppressing this activation reduces rates of podocyte proliferation.44 The fact that TNF-α further exacerbated this persistent NF-κB activation supports the paradigm that HIV can prime the cytokine responsiveness of infected cells.54
Proposed model of inflammatory amplification of nuclear factor-κB (NF-κB) activation in podocytes during the pathogenesis of proliferative podocytopathies. Normal podocytes at steady state have low levels of NF-κB activation, with the majority of NF-κB being sequestered in the cytoplasm through binding its cytoplasmic inhibitor IκB. (1) Pro-inflammatory challenges to quiescent podocytes, such as by tumor necrosis factor-α (TNF-α), initiate NF-κB signaling through degradation of IκB and translocation of NF-κB to the nucleus. In collapsing glomerulopathy, human immunodeficiency virus-1 (HIV-1) proteins can also initiate this NF-κB signaling.53 (2) As a result, multiple NF-κB target genes, including cyclin D1, tumor necrosis factor receptor 2 (TNFR2), and various chemokines, are upregulated further priming the inflammatory responsiveness of podocytes. (3) Primed podocytes expressing immunomodulating proteins de novo are now more susceptible to further activation of NF-κB by multiple factors (eg, TNF-α, bacterial lipopolysaccharide (LPS)) either from the circulation, from infiltrating immune cells, or possibly produced from other renal cell types, thereby amplifying NF-κB signaling (4). This reinforces cell-cycle progression (5), contributing to the pathological changes (6) characteristic of proliferative podocytopathies.
This compounding of podocyte injury via NF-κB is not unusual owing to the multiple pathways that activate NF-κB and the large number of NF-κB target genes, which then further amplify NF-κB signaling.10, 11, 12 Virus-induced or somatic mutations causing NF-κB dysregulation, stimulated by feedforward NF-κB loops from cytokine signaling, have long been known to couple and precipitate or accelerate cellular transformation.64 Our microarray gene profiling showed that podocytes respond similarly to TNF-α, upregulating several NF-κB transcription family members among other target genes that together perpetuate further activation of NF-κB. In summary, these results add to that of others identifying the TNF-α–TNFR2 axis as a compelling pathway for further study of pathogenic NF-κB signaling in proliferative podocytopathies.
References
Van Den Berg JG, Aten J, Chand MA, et al. Interleukin-4 and interleukin-13 act on glomerular visceral epithelial cells. J Am Soc Nephrol 2000;11:413–422.
Lorenzen J, Shah R, Biser A, et al. The role of osteopontin in the development of albuminuria. J Am Soc Nephrol 2008;19:884–890.
Sanchez-Nino MD, Sanz AB, Ihalmo P, et al. The MIF receptor CD74 in diabetic podocyte injury. J Am Soc Nephrol 2009;20:353–362.
Ding M, Cui S, Li C, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 2006;12:1081–1087.
Lee EY, Chung CH, Khoury CC, et al. The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-beta, increases podocyte motility and albumin permeability. Am J Physiol Renal Physiol 2009;297:F85–F94.
Katsuyama K, Fujinaka H, Yamamoto K, et al. Expression of the chemokine fractalkine (FKN/CX3CL1) by podocytes in normal and proteinuric rat kidney glomerulus. Nephron Exp Nephrol 2009;113:c45–c56.
Sayyed SG, Hagele H, Kulkarni OP, et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia 2009;52:2445–2454.
Barisoni L, Nelson PJ . Collapsing glomerulopathy: an inflammatory podocytopathy? Curr Opin Nephrol Hypertens 2007;16:192–195.
Tipping PG . Are podocytes passive or provocative in proteinuric glomerular pathology? J Am Soc Nephrol 2008;19:651–653.
Ghosh S, Hayden MS . New regulators of NF-kappaB in inflammation. Nat Rev Immunol 2008;8:837–848.
Vallabhapurapu S, Karin M . Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 2009;27:693–733.
Rangan G, Wang Y, Harris D . NF-kappaB signalling in chronic kidney disease. Front Biosci 2009;14:3496–3522.
Wiggins RC . The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 2007;71:1205–1214.
Vielhauer V, Mayadas TN . Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol 2007;27:286–308.
Ernandez T, Mayadas T . Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int 2009;76:262–276.
Huugen D, Cohen Tervaert JW, Heeringa P . TNF-alpha bioactivity-inhibiting therapy in ANCA-associated vasculitis: clinical and experimental considerations. Clin J Am Soc Nephrol 2006;1:1100–1107.
Balkwill F . TNF-alpha in promotion and progression of cancer. Cancer Metast Rev 2006;25:409–416.
Aggarwal BB, Shishodia S, Sandur SK, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol 2006;72:1605–1621.
Vielhauer V, Stavrakis G, Mayadas TN . Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest 2005;115:1199–1209.
Al-Lamki RS, Wang J, Vandenabeele P, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J 2005;9:1637–1645.
Moeller MJ, Soofi A, Hartmann I, et al. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 2004;15:61–67.
Thorner PS, Ho M, Eremina V, et al. Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol 2008;19:495–502.
Wilson HM, Chettibi S, Jobin C, et al. Inhibition of macrophage nuclear factor-kappaB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol 2005;167:27–37.
Kim YG, Alpers CE, Brugarolas J, et al. The cyclin kinase inhibitor p.21CIP1/WAF1 limits glomerular epithelial cell proliferation in experimental glomerulonephritis. Kidney Int 1999;55:2349–2361.
Smeets B, Angelotti ML, Rizzo P, et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 2009;20:2593–2603.
Smeets B, Uhlig S, Fuss A, et al. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 2009;20:2604–2615.
Gee K, Kryworuchko M, Kumar A . Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2004;52:13–26.
Koukouritaki SB, Vardaki EA, Papakonstanti EA, et al. TNF-alpha induces actin cytoskeleton reorganization in glomerular epithelial cells involving tyrosine phosphorylation of paxillin and focal adhesion kinase. Mol Med 1999;5:382–392.
Doublier S, Ruotsalainen V, Salvidio G, et al. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 2001;158:1723–1731.
Yamauchi K, Takano Y, Kasai A, et al. Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int 2006;70:892–900.
Hino M, Nagase M, Kaname S, et al. Expression and regulation of adrenomedullin in renal glomerular podocytes. Biochem Biophys Res Commun 2005;330:178–185.
Takano Y, Hiramatsu N, Okamura M, et al. Suppression of cytokine response by GATA inhibitor K-7174 via unfolded protein response. Biochem Biophys Res Commun 2007;360:470–475.
Lai KN, Leung JC, Chan LY, et al. Activation of podocytes by mesangial-derived TNF-{alpha}: glomerulo-podocytic communication in IgA nephropathy. Am J Physiol Renal Physiol 2008;294:F945–F955.
Pawar RD, Castrezana-Lopez L, Allam R, et al. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology 2009;128:e206–e221.
Polzer K, Soleiman A, Baum W, et al. Selective p38MAPK isoform expression and activation in antineutrophil cytoplasmatic antibody-associated crescentic glomerulonephritis: role of p38MAPKalpha. Ann Rheum Dis 2008;67:602–608.
Al-Lamki RS, Wang J, Skepper JN, et al. Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab Invest 2001;81:1503–1515.
Hoffmann U, Bergler T, Rihm M, et al. Upregulation of TNF receptor type 2 in human and experimental renal allograft rejection. Am J Transplant 2009;9:675–686.
Ophascharoensuk V, Pippin JW, Gordon KL, et al. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 1998;54:416–425.
Rosenstiel P, Gharavi A, D’Agati V, et al. Transgenic and infectious animal models of HIV-associated nephropathy. J Am Soc Nephrol 2009;20:2296–2304.
Barisoni L, Madaio MP, Eraso M, et al. The kd/kd mouse is a model of collapsing glomerulopathy. J Am Soc Nephrol 2005;16:2847–2851.
Gherardi D, D’Agati V, Chu TH, et al. Reversal of collapsing glomerulopathy in mice with the cyclin-dependent kinase inhibitor CYC202. J Am Soc Nephrol 2004;15:1212–1222.
Shankland SJ, Pippin JW, Reiser J, et al. Podocytes in culture: past, present, and future. Kidney Int 2007;72:26–36.
Nelson PJ, Sunamoto M, Husain M, et al. HIV-1 expression induces cyclin D1 expression and pRb phosphorylation in infected podocytes: cell-cycle mechanisms contributing to the proliferative phenotype in HIV-associated nephropathy. BMC Microbiol 2002;2:26.
Martinka S, Bruggeman LA . Persistent NF-kappaB activation in renal epithelial cells in a mouse model of HIV-associated nephropathy. Am J Physiol Renal Physiol 2006;290:F657–F665.
Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:RESEARCH0034, 1–11.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001;25:402–408.
Sheehan KC, Pinckard JK, Arthur CD, et al. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J Exp Med 1995;181:607–617.
Saleem MA, Zavadil J, Bailly M, et al. The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. Am J Physiol Renal Physiol 2008;295:F959–F970.
Al-Lamki RS, Wang J, Tolkovsky AM, et al. TL1A both promotes and protects from renal inflammation and injury. J Am Soc Nephrol 2008;19:953–960.
Vandenabeele P, Declercq W, Beyaert R, et al. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol 1995;5:392–399.
Wajant H, Pfizenmaier K, Scheurich P . Tumor necrosis factor signaling. Cell Death Differ 2003;10:45–65.
Albaqumi M, Soos TJ, Barisoni L, et al. Collapsing glomerulopathy. J Am Soc Nephrol 2006;17:2854–2863.
De SK, Devadas K, Notkins AL . Elevated levels of tumor necrosis factor alpha (TNF-alpha) in human immunodeficiency virus type 1-transgenic mice: prevention of death by antibody to TNF-alpha. J Virol 2002;76:11710–11714.
Decrion AZ, Dichamp I, Varin A, et al. HIV and inflammation. Curr HIV Res 2005;3:243–259.
Petermann A, Hiromura K, Pippin J, et al. Differential expression of d-type cyclins in podocytes in vitro and in vivo. Am J Pathol 2004;164:1417–1424.
Guttridge DC, Albanese C, Reuther JY, et al. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 1999;19:5785–5799.
Toualbi-Abed K, Daniel F, Guller MC, et al. Jun D cooperates with p65 to activate the proximal kappaB site of the cyclin D1 promoter: role of PI3K/PDK-1. Carcinogenesis 2008;29:536–543.
Lloyd CM, Dorf ME, Proudfoot A, et al. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukoc Biol 1997;62:676–680.
Hallman TM, Peng M, Meade R, et al. The mitochondrial and kidney disease phenotypes of kd/kd mice under germfree conditions. J Autoimmun 2006;26:1–6.
Gharavi AG, Ahmad T, Wong RD, et al. Mapping a locus for susceptibility to HIV-1-associated nephropathy to mouse chromosome 3. Proc Natl Acad Sci USA 2004;101:2488–2493.
Bruggeman LA, Dikman S, Meng C, et al. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest 1997;100:84–92.
Stevens M, De Clercq E, Balzarini J . The regulation of HIV-1 transcription: molecular targets for chemotherapeutic intervention. Med Res Rev 2006;26:595–625.
Bruggeman LA, Nelson PJ . Controversies in the pathogenesis of HIV-associated renal diseases. Nat Rev Nephrol 2009;5:574–581.
Lu T, Stark GR . Cytokine overexpression and constitutive NFkappaB in cancer. Cell Cycle 2004;3:1114–1147.
Acknowledgements
We thank Volker Vielhauer and Tanya Mayadas for helpful comments and suggestions. This work was supported by the National Institutes of Health Grants DK065498 (PJN), DK079498 (PJN), DK083375 (PJN), and DK061395 (LAB). PJN and LB are members of the Nephrotic Syndrome Study Network (NEPTUNE) funded by Grant DK083912. PED was supported by training Grant DK007470, and LAB is a member of the Case Center for AIDS Research supported by Grant AI36219.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
Rights and permissions
About this article
Cite this article
Bruggeman, L., Drawz, P., Kahoud, N. et al. TNFR2 interposes the proliferative and NF-κB-mediated inflammatory response by podocytes to TNF-α. Lab Invest 91, 413–425 (2011). https://doi.org/10.1038/labinvest.2010.199
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2010.199
Keywords
This article is cited by
-
Inflammatory biomarkers in staging of chronic kidney disease: elevated TNFR2 levels accompanies renal function decline
Inflammation Research (2022)
-
Childhood HIV-associated nephropathy: 36 years later
Pediatric Nephrology (2021)
-
Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA
Nature Communications (2018)
-
Dual neutralization of TNFR-2 and MMP-2 regulates the severity of S. aureus induced septic arthritis correlating alteration in the level of interferon gamma and interleukin-10 in terms of TNFR2 blocking
Immunologic Research (2018)
-
The role of macrophages in hypertension and its complications
Pflügers Archiv - European Journal of Physiology (2017)