Abstract
The protective effects of immunosuppressants against ischemia/reperfusion (I/R) injury have been attributed to their non-specific anti-inflammatory effect. However, these effects may also depend on their effect on T lymphocytes, which are increasingly considered to be key players in I/R. Here, we studied the effects of tacrolimus and sirolimus on lymphocyte subpopulations in an I/R rat model. The animals were treated with tacrolimus, sirolimus or vehicle, before undergoing a 60-min ischemia event of the right hepatic lobe, followed by excision of the remaining liver. After 2 h, I/R rats showed increased mortality, plasma lactate dehydrogenase (LDH) levels, hepatocyte apoptosis, liver histological injury and parenchymal infiltration by neutrophils, macrophages, NK cells and T lymphocytes. Most of the changes were antagonized by both immunosuppressants. Tacrolimus augmented the proportion of cycling cells in I/R rats, whereas sirolimus showed the opposite effect. The increased Th1/Th2 ratio found in I/R livers after 2 h was reverted by immunosuppressants, which also amplified the proportion of CD4+CD25+Foxp3+ regulatory T lymphocytes at 24 h. The protective effects of both tacrolimus and sirolimus correlated well with a decreased ratio of proinflammatory to anti-inflammatory T lymphocytes, and with an increase in the Treg proportion. This suggests a new mechanism to explain the known beneficial effect shown by immunosuppressants early after I/R.
Similar content being viewed by others
Main
Major liver tissue loss associated with periods of ischemia is a common feature in trauma, hepatic surgery and transplantation. The actions of polymorphonuclear leukocytes (PMN) on liver ischemia/reperfusion (I/R) phenomena have been well studied. PMN are considered to be the primary cells responsible for propagating the injury response.1
There is increasing evidence that T lymphocytes, in addition to their well-known role in transplant rejection, are also key players in the acute I/R injury of the graft.2, 3 In fact, they can secrete several cytokines, including TNFα, IFNγ, IL-4 and GM-CSF,4, 5 which could participate in PMN-dependent inflammatory responses. Also, nude, CD4+-depleted, or CD4+- or CD8+-knockout mice suffer a less intense I/R injury in their liver or kidneys than the immunocompetent controls.2, 3 The growing role attributed to lymphocytes in the I/R phenomena would be of particular relevance in the liver, as hepatic parenchyma is especially rich on T lymphocytes and NK cells, which are also known to be carried from the donor to the recipient during liver transplantation.6
Tacrolimus, a widely used immunosuppressant, blocks IL-2 transcription by inhibiting calcineurin. Like most calcineurin inhibitors, besides inhibiting T lymphocytes, it possesses an unspecific anti-inflammatory effect, partly through its action on other defensive cells such as monocytes and PMN.7 This effect has been used to explain the observed beneficial effects of these drugs on liver I/R models.1, 8 However, tacrolimus could also modify the early allocation of T-lymphocyte subpopulations into the liver parenchyma, and this would be an additional mechanism to modulate acute liver inflammation. Thus, we designed this study to determine whether the effect of tacrolimus on an I/R plus hepatectomy model is accompanied by changes in the lymphocyte subpopulations of the liver. Sirolimus does not inhibit calcineurin, but a cell cycle regulatory protein, the mammalian target of rapamycin (mTOR). We also studied whether this specific signaling pathway results in a different effect in our model.
The results of this work show that both tacrolimus and sirolimus have a beneficial effect in liver I/R injury. This effect is accompanied by a short-term decrease in the ratio of proinflammatory to anti-inflammatory T lymphocytes, and by a progressive increase in the proportion of regulatory T lymphocytes, suggesting a common mechanism by which different immunosuppressive drugs could protect the liver early after I/R injury.
MATERIALS AND METHODS
Animals
Sprague–Dawley male rats, weighing 250–300 g, housed and fed under standard laboratory conditions, were used. All animals received humane care, with free access to food and water. They were pretreated with tacrolimus (6 mg/kg, Calbiochem, Darmstadt, Germany) (T), sirolimus (2 mg/kg, Calbiochem) (S) or vehicle (5% propylene glycol and 2% Tween-80 in saline). After 60 min, they underwent a 60-min ischemia event of the right lateral lobe by means of a microvascular clamp. On restoration of flow, the median and left lateral lobes (about 80% of the total liver mass) were excised.9 Other animals underwent hepatectomy alone or a sham operation (Table 1). Enoxaparin (30 mg/kg) was administered subcutaneously 60 min before surgery in all rats.
Animals were killed 2 or 24 h later, and plasma, liver and submaxillary lymph nodes (LN) were obtained. Plasma lactate dehydrogenase (LDH) was determined spectrophotometrically by using a specific kit (Sigma, Spain).
Isolation of Hepatic Lymphoid Cells
Livers were minced into small fragments and washed free of blood in cold PBS. Fragments were passed through a 230-μm steel mesh, and the resulting cell suspension was cotton-filtered to remove tissue debris and resuspended in RPMI-1640 plus 5% FCS. Leukocytes were isolated by density-gradient centrifugation (Histopaque-1077, Sigma). Cell viability was assessed by trypan blue staining.
Flow Cytometric Analysis
Mouse anti-rat mAbs of the following specificities, labeled with FITC, PE, PE-Cy5 or PerCP, were obtained from BD PharMingen (San Diego, CA, USA) and Serotec (Oxford, UK): CD4, CD5, CD8α, CD8β, CD25, CD45RC, NKR-P1A, OX12, TCRαβ and TCRγδ. Three-color flow cytometric analyses were performed using a FACScalibur (BD Biosciences, San Jose, CA, USA), as described earlier.10 For Foxp3-APC staining, a prior cell membrane permeabilization step was performed following the manufacturer's instructions (Cell Fixation/Permeabilization Kit, eBioscience, San Diego, CA, USA).
Histology, Immunofluorescence and Quantification of Apoptosis
Liver cryosections (5–7 μm) were fixed in acetone (immunofluorescence) or paraformaldehyde (Ki67 or TUNEL). After blockade of the unspecific binding with donkey serum (Santa Cruz Biotechnology, Santa Cruz, CA, USA), sections were incubated with His48, anti-CD5, anti-NKR-P1A (PharMingen), ED1, ED2 (European Collection of Animal Cell Cultures) or rabbit anti-laminin (Sigma) antibodies. Liver regeneration was determined using MIB-5 (anti-Ki67) (Dako, Barcelona, Spain), after heat-induced epitope retrieval in citrate buffer. Donkey FITC-conjugated anti-mouse or Texas Red-conjugated anti-rabbit (Jackson Laboratories, West Grove, PA, USA) served as secondary antibodies. Apoptosis was quantified by TUNEL (In situ Cell Death Detection Kit, Roche, Mannheim, Germany). Sections were mounted on Prolong Gold® (Invitrogen, Barcelona, Spain) and examined under a fluorescence microscope, Zeiss Axioplan 2® (Oberkochen, Germany).
Liver sections stained with hematoxylin–eosin were scored using the system devised by Suzuki et al,1 grading the tissue damage from 0 to 4 on the basis of the presence and severity of congestion, cytoplasmic vacuolization and cell necrosis.
For quantitative determinations, 10 non-overlapping fields were analyzed in a blinded manner for each of a minimum of six animals per group. They were randomly selected from four liver sections at least 1500 μm apart. A proportion of studied cells were quantified by the ImageJ® software (NIH, Bethesda, MD, USA), determining the positive cells/total nuclei (Hoechst-33342®, Molecular Probes, Eugene, OR, USA) ratio in each field.
Statistical Analysis
Results are expressed as means (±s.e.m.) of at least six independent experiments. Comparison of means was performed by ANOVA (Tukey as post-hoc test), and survival analysis by the log-rank method (Statgraphics Centurion® software).
RESULTS
Both Tacrolimus and Sirolimus Increase Survival and Decrease Hepatocellular Injury Among I/R Rats
Initially we studied rat survival and hepatocellular injury, the latter through the analysis of plasma LDH levels, histological damage and apoptosis rate. Eight out of 20 animals in the I/R group died, whereas there was no mortality in the sham or hepatectomy groups. Tacrolimus or sirolimus treatment decreased both mortality and plasma LDH in the I/R group (Figure 1a and b).
Animal survival and hepatocellular injury are improved by tacrolimus and sirolimus. (a) Survival curves of rats from the different ischemia/reperfusion (I/R) groups (N=20 rats/group), compared according to log-rank (Mantel–Cox) test. Tacrolimus and sirolimus increase the survival of rats undergoing I/R injury. (b) Both drugs antagonize the increase in plasma lactate dehydrogenase (LDH) levels showed by I/R rats killed at 2 h. (c, d) Hematoxylin-eosin-stained cryosections of I/R livers (d) showed signs of tissue damage, such as congestion (*), cytoplasmic vacuolization (inset) and necrosis (arrow), when compared with control livers (c). These lesions were quantified by Suzuki's score (e). There was a decrease in the tissue damage score at 2 h in treated I/R rats. (f–h) Liver samples from the different groups were collected for TUNEL and the nuclei were detected by Hoechst 33342 to quantify the proportion of apoptotic cells. The photographs show homogeneous distribution of the positive cells representative of liver cryosections from I/R (f), I/R+T (g), and I/R+S (h) groups. The inset photograph in (f) shows the restricted nuclear staining obtained for TUNEL. Both drugs markedly reduced the apoptosis rate found among the hepatocytes. §P<0.05 vs the rest; *P<0.05 vs control; ##P<0.01 vs the other untreated controls. (c, d) Original magnification × 200 (insert, × 1000). (f–h) Original magnification × 100 (insert, × 1000). Each data point in b and e graphs represents the mean and s.e. of values found in at least six animals. PA: portal area; CV: central vein; +T: plus tacrolimus treatment; +S: plus sirolimus treatment.
Rats killed 2 or 24 h after I/R showed severe cytoplasmic vacuolization, sinusoidal congestion and parenchymal necrosis (Figure 1c and d), whereas milder changes were appreciable in the immunosuppressant pretreated rats. When quantified using the Suzuki's score,1 the damage amelioration showed statistical significance already at 2 h (Figure 1e). The hepatocyte apoptosis rate detected by TUNEL was also higher after I/R (64.0±4.8 per 1000) than after sham operation (0.73±0.11, P<0.01) or hepatectomy (7.5±0.8, P<0.01). It was again markedly decreased by tacrolimus or sirolimus treatment (33.4±3.7 and 26.8±1.8, respectively, both P<0.01 vs I/R control) (Figure 1f–h).
Collectively, these data show an early beneficial effect of both immunosuppressants in decreasing hepatocellular damage and rat mortality.
Both Drugs Display Opposite Effects on Liver Regeneration
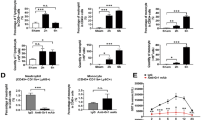
The liver's capability to restore the hepatic functional mass could influence the consequences of I/R injury. Thus, a liver regeneration index and the effects of tacrolimus and sirolimus on this parameter were analyzed in our model. Hepatocyte proliferation was increased in all hepatectomy and I/R rats as early as 2 h after the procedure. At 24 h, tacrolimus increased further the rate of cycling cells, whereas sirolimus decreased it (Figure 2).
The hepatocyte proliferation rate experiences an opposite behavior after drug treatments. Ki-67+ proliferating hepatocytes were determined at 2 (a) and 24 (b) hours after the interventions by immunofluorescence contrasted with Hoechst 33342 and laminin. The graphs represent the values obtained from different rats group showing that hepatectomy and ischemia/reperfusion (I/R) trigger an increase in hepatocyte proliferation, which is augmented by tacrolimus and reduced by sirolimus at 24 h. Each bar in the graphs represents the mean and s.e. of values found in at least six animals. *P<0.05 vs its control; **P<0.01 vs its control; ##P<0.01 vs the other untreated controls; §§P<0.01 vs sham control.
Treated I/R Rats Show Less Infiltration by Inflammatory Cells
The I/R phenomena and the ensuing hepatocellular damage activated an inflammatory response characterized by a massive leukocyte infiltration, consisting mainly of PMN and monocytes, and also of lymphocytes and NK cells.
Liver neutrophils (HIS48+ cells) increased clearly at 2 h after I/R, both tacrolimus and sirolimus being very effective in attenuating PMN infiltration (Figure 3). At 24 h, the infiltrating PMN were mainly confined to the areas of necrosis and intergroup differences were difficult to quantify.
Decreased hepatic neutrophil recruitment in ischemia/reperfusion (I/R) after tacrolimus or sirolimus administration. Three-color immunofluorescence analyses (b–d) were carried out to determine the proportion (a) and distribution of polymorphonuclear leukocytes (PMN, HIS-48+ cells) in the liver parenchyma. Liver cryosections were marked with anti-laminin antibody and anti-HIS 48, and the nuclei were labeled with Hoechst 33342. The photographs (b–d) are representative of immunofluorescence staining obtained in livers from rats of the I/R (b), I/R+T (c) and I/R+S (d) groups killed 2 h after the intervention. The PMN appear homogeneously distributed through the parenchyma in all groups, but their number was drastically increased in the untreated I/R rats (b). Original magnification × 200. Each bar in graph represents the mean and s.e. of values found in at least six animals killed at 2 h. **P<0.01 vs its control; ##P<0.01 vs the other controls; §P<0.05 vs sham control. PA: portal area.
Although the number of macrophage/Kupffer (ED2+) cells did not differ between groups, that of monocytes/immature macrophages (ED1+) increased after I/R (30.4±3.6, I/R, vs 17.7±2.8, sham, 2 h, and 27.4±3.3, I/R, vs 15.6±2.5, sham, 24 h, per 1000, P<0.05 for both). Immunosuppressants showed no effect on these cell populations.
Both hepatectomy and I/R rats showed an increased NK cell number (18.5±2.1, I/R, and 10.6±1.0, hepatectomy, vs 3.2±0.6, sham, per 1000, P<0.01 for both), mainly located in and around the portal areas (Figure 4a). Such increase was partially reversed in pretreated I/R rats, being significant for sirolimus (to 7.5±0.9, P<0.05) (Figure 4b and c). Intrahepatic NK cells returned to baseline at 24 h in all groups.
Both sirolimus and tacrolimus decreased ischemia/reperfusion (I/R)-induced hepatic infiltration by NK and T cells. Hepatic cryosections from different I/R groups, killed 2 h after reperfusion, were stained for laminin (expressed in the basal lamina of blood vessels), nuclei (Hoechst 33342), and NK cells (anti-NKR-P1A) (photographs a–c ) or T cells (anti-CD5) (photographs d, e ). A detail of a cell with NKR-P1A positive staining is showed in the inset in (a). Control I/R livers show a higher proportion of both NK and T cells, distributed mainly on the portal areas (PA) or in the parenchyma, respectively, than the groups treated with tacrolimus or sirolimus. The images are representative examples of at least six animals per group. Original magnifications (a–f) × 200 and inset × 1000. +T: plus tacrolimus treatment; +S: plus sirolimus treatment.
I/R induced an increase in parenchymal T cells at 2 h (15.8±2.5, I/R, vs 4.6±0.4, hepatectomy, and 2.9±0.4, sham, per 1000, P<0.01 for both) and 24 h (5.9±1.3, I/R, vs 2.8±0.4, hepatectomy, and 2.0±0.3, sham, per 1000, P<0.05 for both). This increase was attenuated by both immunosuppressants (5.6±0.7, I/R+T, and 9.5±0.8, I/R+S, per 1000, 2 h, P<0.05 for both).
Therefore, tacrolimus or sirolimus can attenuate the increase in hepatic tissue infiltration by PMN, NK and T cells.
Immunosuppressants Modulate I/R-Induced Changes in Several Lymphocyte Subpopulations
A cytofluorometric analysis of the hepatic lymphocytes was performed to complement the immunofluorescence findings. In addition, we studied the submaxillary LN subpopulations to help distinguish a local from a systemic response.
Regarding NK and NKT cells, their proportions were higher in the liver than in the LN (Figure 5a), although without intergroup differences.
Changes in NK cells, and B and Tγδ lymphocyte populations, isolated from liver samples. Different leukocyte subpopulations, obtained from lymph node (LN) and liver of the different groups of rats as indicated, were analyzed by cytofluorimetric analysis of NK cells, and B and Tγδ lymphocyte markers. (a) The dot plots represent the NKR-P1A and TCRαβ expression on gated lymphocytes from LN and liver of sham control rats killed at 2 and 24 h as indicated, exhibiting a higher proportion of classical NK (NKR-P1A+ TCRαβ−) and NKT (NKR-P1A+ TCRαβ+) cells in liver parenchyma. (b) Proportions of total B (OX12+) and B1 (OX12+CD5+), and TCRγδ lymphocytes from the liver of different groups of rats killed at 2 and 24 h, as indicated. The graphs represent the mean and s.e. of values obtained from isolated hepatic lymphocytes of at least six animals, and they evidence an effect of both drugs in decreasing total B and Tγδ lymphocytes, and in reverting the ischemia/reperfusion (I/R)-induced increase of B1 cells. *P<0.05 vs its control; **P<0.01 vs its control; ##P<0.01 vs other untreated controls. +T: plus tacrolimus treatment; +S: plus sirolimus treatment; h: hours.
The proportion of total B lymphocytes was not influenced by hepatectomy or I/R, although it was decreased by both drugs at 2 h (Figure 5b). However, the B1 (OX12+CD5+) subpopulation increased at 2 and 24 h after I/R (Figure 5b), being antagonized by tacrolimus or sirolimus pretreatment.
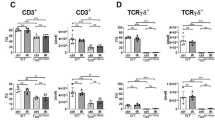
The main subpopulations of T lymphocytes , including Tαβ CD4 and CD8 cells, as well as Tγδ cells, were also analyzed. Liver Tγδ cells were not modified by hepatectomy or I/R, although both drugs decreased their proportion at 24 h. The effect of sirolimus was already evident at 2 h (Figure 5b).
The hepatic proportion of TCRαβ+ cells experienced an early increase after I/R in all groups, with a parallel decrease in the LN, whereas no changes were detected at 24 h (Figure 6). At 2 or 24 h, we found no intergroup differences in the proportions of CD4 or CD8 cells (Figure 6).
T lymphocyte proportion underwent a transient increase in the liver after ischemia/reperfusion (I/R). The proportion of lymphocyte Tαβ subpopulations was determined by flow cytometry. CD4 vs CD8α expression (dot plots) was analyzed on gated TCRαβ cells (histograms) from liver and lymph node at different intervals after reperfusion as indicated. A discrete shift in the proportion of T cells from the lymph node to the liver, without changes in CD4 or CD8 subpopulations, is appreciated in I/R groups at 2 h. Data on the plots are representative of at least six independent experiments.
We also analyzed liver and LN lymphocytes for other less prevalent T-cell subpopulations: immature CD4+CD8+ and extrathymically differentiated CD8αα lymphocytes, which are increased in several pathological situations. Neither hepatectomy nor I/R modified these subpopulations, which also remained unchanged in the drug pretreated groups (Figure 7).
Double-positive and CD8αα T lymphocytes are almost absent in the liver. Intrahepatic lymphocytes of different group rats, killed at 2 and 24 h, were stained with anti-TCRαβ, anti-CD4, anti-CD8α and anti-CD8β antibodies for cytofluorimetric analysis. The left histogram shows the expression of TCRαβ in the total hepatic lymphocyte population. The dot plots represents the CD4 vs CD8α (upper row) and the CD8α vs CD8β (lower row) expression among TCRαβ+ lymphocytes, showing that double-positive (CD4+CD8α+) or CD8αα (CD8α+CD8β−) T lymphocytes neither reach important proportions nor change after ischemia/reperfusion (I/R). Data on the plots are representative of at least six rats of the indicated groups killed at 24 h, and similar plots were obtained from rats of the remaining groups.
Therefore, the subpopulations most affected by tacrolimus or sirolimus pretreatment are B, B1 and Tγδ cells.
Both Drugs Palliate the I/R-Induced Increase in Th1/Th2 Ratio
Depending on their pattern of secreted cytokines, two main classes of CD4 T lymphocytes are distinguished: Th1 and Th2, with pro- and anti-inflammatory properties, respectively. Both subpopulations differ in their CD45RC expression level.11 Among hepatic TCRαβ+CD4+ lymphocytes, the ratio of proportion of Th1 (CD45RChigh) cells to that of Th2 (CD45RClow) cells was increased 2 h after I/R, a finding that was antagonized by both drugs (Figure 8). However, these changes were not observed in animals killed at 24 h, neither were they mirrored in the submaxillary LN, suggesting a local, acute, short-term response.
Liver parenchyma Th1/Th2 CD4 T cell proportions are modulated by immunosuppressive drugs. Liver TCRαβ+CD4+ lymphocytes were analyzed for CD45RC expression by flow cytometry to determine their Th1 (CD45RChigh) or Th2 (CD45RClow) phenotype. The relative proportions of Th1 and Th2 among CD4 T lymphocytes are represented in a stacked bar chart, which displays the mean values obtained from isolated hepatic lymphocytes of at least six animals in the different groups as indicated. There is a significant increase in the Th1/Th2 proportion after ischemia/reperfusion (I/R), which is partially reverted by both immunosuppressants. H: hepatectomy; +T: plus tacrolimus treatment; +S: plus sirolimus treatment.
Treg Cells Experienced a Late Increase in Treated Groups
Another T-lymphocyte subpopulation, the Treg, is being increasingly involved in modulating diverse inflammatory responses. Regulatory T lymphocytes are characterized by their expression of CD25 and the transcriptional repressor Foxp3.12 Although we found no changes in the proportion of CD4+CD25+FoxP3+ cells in the liver or LN at 2 h, tacrolimus and sirolimus increased the Treg percentage at 24 h after I/R, whereas sirolimus showed a similar effect also in hepatectomy and sham groups (Figure 9).
Local increase of regulatory T lymphocytes in the liver parenchyma. (a) The CD25 and Foxp3 markers were used to study the regulatory T lymphocytes in gated TCRαβ+CD4+ population (right dot plot). The left dot plot shows a direct correlation between the expression of both markers, allowing us to define regulatory T lymphocytes as CD25+Foxp3+ among the TCRαβ+CD4+ population. (b, c) The bar graphs represent the proportion of regulatory T cells among the CD4 T lymphocytes of livers (b), or lymph nodes (LN) (c), at 24 h after reperfusion. Each bar in graphs represents the mean and s.e. of values found in at least six animals per group. *P<0.05 vs control; **P<0.01 vs control. I/R: ischemia/reperfusion.
DISCUSSION
Reperfusion injury plays an important role in the development of primary nonfunctioning of the liver graft after transplantation, having an incidence ranging from 6 to 9.2%,13, 14 and requiring liver retransplantation for the patient to survive. In this study, just 8 out of 20 untreated I/R rats died; thus, our model might resemble a less severe condition, but it permits enough sensitivity for assessing changes in the animal survival rate.
T lymphocytes are increasingly considered to participate in the inflammatory injury secondary to I/R,2, 15, 16 although their implication is not completely out of question.17 Attachment of circulating lymphocytes to the hepatic sinusoids has been observed early after the reperfusion,18 which could explain why we found them increased in the liver parenchyma as early as 2 h. In addition, a human liver contains about 1010 lymphocytes, and passenger lymphocytes seem to play a key pathogenic role in the reperfusion that occurs after cold ischemia of the liver.16 Nevertheless, the role of these resident lymphocytes vs peripheral ones or the interrelation between both populations remains unknown.
The effect of T lymphocytes is probably multifactorial as suggested by the fact that most systemic immunosuppressants attenuate this injury.15 Although both CD4+ and CD8+ T lymphocytes seem to contribute to the microvascular damage after intestinal I/R,18 one key aspect would be the action of CD4+ lymphocytes as a determinant of the subacute PMN infiltration.2 This PMN recruitment might depend on a direct action of CD4+ lymphocytes, through their capacity to produce several cytokines,4, 18 or in an indirect way, through the stimulus to produce chemotactic factors by Kupffer cells.15 However, a beneficial effect has also been observed with peripheral T-lymphocyte depletion, without concomitant modifications in hepatic PMN, during I/R injury.19 Thus, the pathogenic role of T lymphocytes does not seem to be limited to PMN recruitment, and may involve other lymphoid populations.
B lymphocytes have also been implicated in the I/R injury.20 Although we found no changes in total B lymphocytes, the B1 subpopulation, which was found pathogenic after intestinal reperfusion,21 experienced an early increase after I/R. Plausibly, other lymphocyte subpopulations also related to innate immunity, including Tγδ and NKT cells, could play a role in I/R, which is considered to be an antigen-independent condition.22 Tγδ lymphocytes can survey, among other antigens, heat-shock proteins and modified cell components, and they seem to play an important role in the early stages of systemic inflammatory response syndrome and sepsis.23 However, the higher level of aggression of our hepatectomy and I/R groups did not result in local or systemic changes in the TCRγδ proportion.
In a variety of conditions, including organ transplantation,24 a premature exit of the thymus of DP cells has been reported; however, the presence of these cells in our model was small, discarding changes in the hematothymic barrier.10 We also ruled out an augmented extrathymic T-cell differentiation by determining TCR+CD8αα lymphocytes, whose peripheral proportion had been reported to increase in some pathological situations, including liver injury.25
In this study, both tacrolimus and sirolimus showed a beneficial effect by reducing the mortality of I/R animals. The sirolimus dose (2 mg/kg) was confined within the range employed experimentally for immunosuppression or graft tolerance in rats. When administered as a single dose, rodent studies have shown a dose–response relationship with allograft survival. Sirolimus doses of 2–6 mg/kg/day provided maximum graft survival in the heterotopic heart allograft model,26 and intraperitoneal doses as high as 24 mg/kg have been used to induce tolerance to skin allograft in mice.27, 28 The tacrolimus dose employed by us (6 mg/kg) was also near or within the range that has been used in experimental models of allograft rejection29, 30, 31 or of the injury induced after reperfusion32 or partial hepatectomy.33 In addition, some studies suggest that a higher than usual dose of tacrolimus is required for inhibiting the innate cell-mediated immune response, and doses of 10 mg/kg/day have been used in mice for this purpose.34, 35 Yet, it is difficult to judge whether both drugs have been administered in equivalent doses, because of the large amplitude of the dosage range used in animal models, their different mechanism of action and their uneven spectrum of collateral effects.
The protective effects of tacrolimus and sirolimus observed here were accompanied by a decrease, not only in the area of necrosis, but also in the percentage of apoptotic cells. Necrosis is typically the consequence of an acute metabolic disturbance, whereas apoptosis represents the execution of a programmed sequence, often triggered by a specific stimulus. Both TNFα36 and IL-437 have been shown to trigger hepatocyte apoptosis. Furthermore, these and other mediators can be released by infiltrating lymphocyte subpopulations, some of which could also upregulate their Fas-L or TLR-2 expression, which are also known to trigger hepatocyte apoptosis.38 Therefore, apoptosis might be a more sensitive parameter than necrosis in measuring the effect of immunosuppressive drugs, otherwise supposed to exert their action primarily on lymphocytes. The distinction between necrosis and apoptosis can be in fact artificial, as the necrosis, in certain circumstances, can be considered to be an abortive apoptosis because of their being insufficient ATP to complete the apoptotic program.39 Molecules with known mitochondrial protective capacity, including tacrolimus,40 also protect against apoptosis.41 I/R plus hepatectomy is a particularly energy-demanding situation, and preventing mitochondrial dysfunction may contribute to the beneficial effect of tacrolimus, especially when this effect seems to be independent of the immunosuppressive action.42 In contrast, sirolimus, by activating procaspase-3, can induce apoptosis,43 which may explain the slight apoptosis increase we found at 24 h (data not shown), although the protective properties of sirolimus seem to prevail at 2 h.
The stimulating effect of tacrolimus and other calcineurin inhibitors on hepatic regeneration is well known,44, 45 although the mechanism is far from clear.44, 45 On the other hand, our and others’ results show an abundance of NK and NKT cells in the liver relative to other lymphoid organs.46 These cells exert a toxic effect on cycling hepatocytes, resulting in inhibition of liver regeneration as a net effect.44, 47 Therefore, a possible hypothesis is that the inhibitors of calcineurin could operate through the inhibition44, 45 or elimination48 of intrahepatic NK cells. Tacrolimus can inhibit the expansion of NKT cells in mice undergoing partial hepatectomy,33 and our results show a decreased NK cells liver infiltrate in tacrolimus-treated groups. However, sirolimus, which inhibited regeneration, also decreased NK cells, arguing against the hypothesis of an indirect, NK- or NKT-mediated, effect of tacrolimus in stimulating hepatocyte regeneration.
Both decrease of hepatic injury and increase of regeneration might be involved in the beneficial effect of tacrolimus. Sirolimus, however, induced a profound inhibition of hepatocyte regeneration. To force hepatocytes to divide can compromise the capacity of a limited residual hepatocyte mass to cope with increased metabolic demands. Thus, both stimulus and inhibition of the hepatic regeneration can be beneficial, but it seems more probable that this could be an effect of relatively minor importance for the outcome. Therefore, the decrease in mortality found in our model might well have been entirely because of alternative mechanisms of protection.
Our data show that a great number of T lymphocytes are recruited in the liver early after injury, further supporting a role for these cells in the early unspecific damage ensuing after I/R. The effect of immunosuppressants in reducing the intrahepatic number of T lymphocytes may have contributed to their protective effect, probably in part by decreasing PMN accumulation. Besides the known effect of these drugs on the proliferation of T cells, this decreased lymphocyte infiltrate might be partly because of the suppressing effect of calcineurin inhibitors on the endothelial adhesion of lymphocytes through the route VLA-4/VCAM-1.49 Similar mechanisms could have contributed in treated rats to the observed decrease of B and Tγδ cells, although we cannot know their relative contribution to the protective action of both drugs in the I/R injury.
The effector mechanisms underlying CD4+ T cell-mediated tissue injury are obscure. These lymphocytes could be activated by chemokines and cytokines released by other infiltrating cells, and proinflammatory molecules can drive the naïve T cells to a more aggressive phenotype.50 CD4+ T lymphocytes have been functionally differentiated into Th1 cells that produce INFγ, lymphotoxin and TNFα, and Th2 cells that produce IL-4, IL-5, IL-6, IL-10 and IL-13.51 An inflammatory pattern of Th1 predominance seems pathogenic, whereas a polarized Th2 pattern may be protective against I/R injury.50, 52 Although the precocity of the Th1/Th2 response in our model is noteworthy , a negative correlation between parameters of liver injury and expression of Th2 cytokines has been observed in humans already at 90 min after reperfusion of the liver.53 Th1/Th2 changes disappeared in animals killed at 24 h, suggesting an acute, short-term effect. Neither were these changes mirrored in the submaxillary LN, indicating also a compartmentalization in the response; therefore, the ability we found for tacrolimus and sirolimus to antagonize the early increase in Th1/Th2 ratio could have been more important in reducing the hepatocellular injury than to modify the total number of CD4+ T cells.
Regulatory T lymphocytes can suppress the response not only of other effector lymphocytes,54 but also of dendritic cells and macrophages,55 and they may play a role in nonspecific inflammatory reactions. A systemic amplification of the Treg population has been reported after severe injury and it might contribute to the post-injury immunosuppression.56 Sirolimus can expand peripheral CD4+CD25+ cells and their regulatory capacity in vivo,57 as well as the selective survival of these cells in vitro.58 As the specificity of location into the inflamed organ seems crucial for Treg cells to exert their function,59 the increased proportion of CD4+CD25+Foxp3+ cells only in the liver adds further support to the hypothesis that sirolimus exerts its protective effect partly by this mechanism. In contrast, calcineurin inhibitors have been reported to induce a peripheral depletion in Treg cells,60, 61 and to suppress their function.62 The reason for such a divergent effect could be the well-known differences between calcineurin inhibitors and m-TOR inhibitors regarding their action on IL-2,62 the signaling through this interleukin being crucial for the activity of CD4+CD25+Foxp3+ cells.63 Tacrolimus decreased Treg cells at the LN in I/R rats, but, interestingly, their proportion was increased in the liver.
In summary, the protection achieved by both tacrolimus and sirolimus in hepatic I/R is accompanied by a decrease of hepatic T lymphocytes and by complex changes in the relative proportions of lymphocyte subpopulations. The net effect would be not only a decrease in the main effector cells, but also an increase of those lymphocyte subpopulations with regulatory activity—an early decrease in the Th1/Th2 balance and a progressive increase in the Treg lymphocytes. This suggests that, despite their different signaling pathways, both drugs modify the early-local immune response in a common way. It also opens a door to future studies of new therapeutic strategies against the I/R injury directed at harnessing lymphoid subpopulations with regulatory capability instead of the traditional strategy to neutralize/eliminate effector cells or their products.
References
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, et al. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993;55:1265–1272.
Zwacka RM, Zhang Y, Halldorson J, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest 1997;100:279–289.
Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 2001;108:1283–1290.
Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 1991;254:279–282.
Khandoga A, Hanschen M, Kessler JS, et al. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology 2006;43:306–315.
Navarro F, Portales P, Candon S, et al. Natural killer cell and alphabeta and gammadelta lymphocyte traffic into the liver graft immediately after liver transplantation. Transplantation 2000;69:633–639.
Arias-Diaz J, Villa N, Hernandez J, et al. Inhibition of arachidonic acid metabolism may mediate the cyclosporine A effect on human pulmonary macrophages. Br J Surg 1996;83:78–79.
Sakr MF, Zetti GM, Hassanein TI, et al. FK 506 ameliorates the hepatic injury associated with ischemia and reperfusion in rats. Hepatology 1991;13:947–951.
Landa I, Arias-Diaz J, Gomez M, et al. Cytoprotective effect of somatostatin in a rat model of hepatic ischemic reperfusion. Hepatology 1992;16:1474–1476.
Jimenez E, Sacedon R, Vicente A, et al. Rat peripheral CD4+CD8+ T lymphocytes are partially immunocompetent thymus-derived cells that undergo post-thymic maturation to become functionally mature CD4+ T lymphocytes. J Immunol 2002;168:5005–5013.
Subra JF, Cautain B, Xystrakis E, et al. The balance between CD45RChigh and CD45RClow CD4 T cells in rats is intrinsic to bone marrow-derived cells and is genetically controlled. J Immunol 2001;166:2944–2952.
Fehervari Z, Sakaguchi S . Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol 2004;16:203–208.
Busuttil RW, Farmer DG, Yersiz H, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg 2005;241:905–916; discussion 916–918.
Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4000 consecutive patients at a single center. Ann Surg 2000;232:490–500.
Matsuda T, Yamaguchi Y, Matsumura F, et al. Immunosuppressants decrease neutrophil chemoattractant and attenuate ischemia/reperfusion injury of the liver in rats. J Trauma-Inj Infect Crit Care 1998;44:475–484.
Le Moine O, Louis H, Demols A, et al. Cold liver ischemia-reperfusion injury critically depends on liver T cells and is improved by donor pretreatment with interleukin 10 in mice. Hepatology 2000;31:1266–1274.
Park P, Haas M, Cunningham PN, et al. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am J Physiol Ren Physiol 2002;282:F352–F357.
Shigematsu T, Wolf RE, Granger DN . T-lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia-reperfusion. Microcirculation 2002;9:99–109.
Anselmo DM, Amersi FF, Shen X-D, et al. FTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltration. Am J Transplant 2002;2:843–849.
Burne-Taney MJ, Ascon DB, Daniels F, et al. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 2003;171:3210–3215.
Reid RR, Woodcock S, Shimabukuro-Vornhagen A, et al. Functional activity of natural antibody is altered in Cr2-deficient mice. J Immunol 2002;169:5433–5440.
Lappas CM, Day Y-J, Marshall MA, et al. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 2006;203:2639–2648.
Matsushima A, Ogura H, Fujita K, et al. Early activation of gammadelta T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock 2004;22:11–15.
Godden U, Herbert J, Stewart RD, et al. A novel cell type carrying both Th and Tc/s markers in the blood of cyclosporine-treated, allografted rats. Transplantation 1985;39:624–628.
Abo T, Kawamura T, Watanabe H . Physiological responses of extrathymic T cells in the liver. Immunol Rev 2000;174:135–149.
DiJoseph JF, Fluhler E, Armstrong J, et al. Therapeutic blood levels of sirolimus (rapamycin) in the allografted rat. Transplantation 1996;62:1109–1112.
Anam K, Akpinar E, Craighead N, et al. Targeted T-cell depletion or CD154 blockade generates mixed hemopoietic chimerism and donor-specific tolerance in mice treated with sirolimus and donor bone marrow. Transplantation 2004;78:1290–1298.
Hale DA, Gottschalk R, Maki T, et al. Determination of an improved sirolimus (rapamycin)-based regimen for induction of allograft tolerance in mice treated with antilymphocyte serum and donor-specific bone marrow. Transplantation 1998;65:473–479.
Toyofuku A, Yasunami Y, Nabeyama K, et al. Natural killer T-cells participate in rejection of islet allografts in the liver of mice. Diabetes 2006;55:34–39.
Behbod F, Erwin-Cohen RA, Wang ME, et al. Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J Immunol 2001;166:3724–3732.
Fealy MJ, Umansky WS, Bickel KD, et al. Efficacy of rapamycin and FK 506 in prolonging rat hind limb allograft survival. Ann Surg 1994;219:88–93.
Hackert T, Pfeil D, Hartwig W, et al. Ciclosporin aggravates tissue damage in ischemia reperfusion-induced acute pancreatitis. Pancreas 2006;32:145–151.
Kato T, Sato Y, Takahashi S, et al. Involvement of natural killer T cells and granulocytes in the inflammation induced by partial hepatectomy. J Hepatol 2004;40:285–290.
Asano K, Taki M, Matsuo S, et al. Mode of action of FK-506 on protective immunity to Hymenolepis nana in mice. In Vivo 1996;10:537–545.
Yoshikai Y, Kidokoro H, Kimura K, et al. Clonal expansion of superantigen-reactive T cells is resistant to FK506 in mice with AIDS. J Virol 1997;71:746–749.
Kuhla A, Eipel C, Siebert N, et al. Hepatocellular apoptosis is mediated by TNFalpha-dependent Fas/FasLigand cytotoxicity in a murine model of acute liver failure. Apoptosis 2008;13:1427–1438.
Aoudjehane L, Podevin P, Scatton O, et al. Interleukin-4 induces human hepatocyte apoptosis through a Fas-independent pathway. FASEB J 2007;21:1433–1444.
Renna MS, Correa SG, Porporatto C, et al. Hepatocellular apoptosis during Candida albicans colonization: involvement of TNF-alpha and infiltrating Fas-L positive lymphocytes. Int Immunol 2006;18:1719–1728.
Malhi H, Gores GJ, Lemasters JJ . Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 2006;43:S31–S44.
Kaibori M, Inoue T, Tu W, et al. FK506, but not cyclosporin A, prevents mitochondrial dysfunction during hypoxia in rat hepatocytes. Life Sci 2001;69:17–26.
Li J, Bombeck CA, Yang S, et al. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J Biol Chem 1999;274:17325–17333.
Waldmeier PC, Feldtrauer JJ, Qian T, et al. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol 2002;62:22–29.
Zhang JF, Liu JJ, Lu MQ, et al. Rapamycin inhibits cell growth by induction of apoptosis on hepatocellular carcinoma cells in vitro. Transplant Immunol 2007;17:162–168.
Tamura F, Masuhara A, Sakaida I, et al. FK506 promotes liver regeneration by suppressing natural killer cell activity. J Gastroenterol Hepatol 1998;13:703–708.
Tanaka N, Yamamoto H, Tatemoto A, et al. Regulation of liver regeneration by interleukin-2 and its inhibitors: cyclosporine A and FK 506. Int J Immunopharmacol 1993;15:211–218.
Racanelli V, Rehermann B . The liver as an immunological organ. Hepatology 2006;43:S54–S62.
Ito H, Ando K, Nakayama T, et al. Role of Valpha 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology 2003;38:1116–1124.
Oishi K, Hayamizu K, Aihaiti X, et al. G-CSF-induced evacuation of sinusoidal NK cells and the facilitation of liver regeneration in a partial hepatectomy. Cytokine 2006;34:66–75.
Tsuzuki S, Toyama-Sorimachi N, Kitamura F, et al. FK506 (tacrolimus) inhibits extravasation of lymphoid cells by abrogating VLA-4/VCAM-1 mediated transendothelial migration. FEBS Lett 1998;430:414–418.
Ysebaert DK, De Greef KE, De Beuf A, et al. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 2004;66:491–496.
Abbas AK, Murphy KM, Sher A . Functional diversity of helper T lymphocytes. Nature 1996;383:787–793.
Yokota N, Burne-Taney M, Racusen L, et al. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 2003;285:F319–F325.
Pulitano C, Sitia G, Aldrighetti L, et al. Reduced severity of liver ischemia/reperfusion injury following hepatic resection in humans is associated with enhanced intrahepatic expression of Th2 cytokines. Hepatol Res 2006;36:20–26.
Coombes JL, Robinson NJ, Maloy KJ, et al. Regulatory T cells and intestinal homeostasis. Immunol Rev 2005;204:184–194.
Murphy TJ, Choileain NN, Zang Y, et al. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol 2005;174:2957–2963.
Ni Choileain N, MacConmara M, Zang Y, et al. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol 2006;176:225–236.
Battaglia M, Stabilini A, Roncarolo M-G . Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005;105:4743–4748.
Strauss L, Whiteside TL, Knights A, et al. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol 2007;178:320–329.
Siegmund K, Feuerer M, Siewert C, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 2005;106:3097–3104.
Segundo DS, Ruiz JC, Izquierdo M, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 2006;82:550–557.
Noris M, Casiraghi F, Todeschini M, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol 2007;18:1007–1018.
Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 2006;108:390–399.
Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005;6:1142–1151.
Acknowledgements
We thank Angeles Vicente, Rosa Sacedón and Marina Guerin for their valuable suggestions, Pablo González and Cruz Rodríguez-Bobada for their help with the animal procedures and care, Carmen Hernández and Amalia Vázquez for their assistance with the cytometry analyses, and Cati Escribano and Virginia Peinado for their help with the H–E staining. This work was supported by grants BFU2004–03132, BFU2007-65520, GR74/07-910552 and RD06/0010/0003 from the Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arias-Diaz, J., Ildefonso, J., Muñoz, J. et al. Both tacrolimus and sirolimus decrease Th1/Th2 ratio, and increase regulatory T lymphocytes in the liver after ischemia/reperfusion. Lab Invest 89, 433–445 (2009). https://doi.org/10.1038/labinvest.2009.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2009.3
Keywords
This article is cited by
-
RORγt inverse agonist TF-S14 inhibits Th17 cytokines and prolongs skin allograft survival in sensitized mice
Communications Biology (2024)
-
Tacrolimus ameliorates thrombocytopenia in an ITP mouse model
Annals of Hematology (2020)
-
Migration of splenic lymphocytes promotes liver fibrosis through modification of T helper cytokine balance in mice
Journal of Gastroenterology (2015)
-
Sirolimus Attenuates Reduced-Size Liver Ischemia–Reperfusion Injury But Impairs Liver Regeneration in Rats
Digestive Diseases and Sciences (2010)
-
Regulatory T cells: a brake on ischemic injury or an active promoter of tissue healing?
Kidney International (2009)