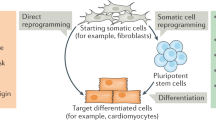
Abstract
Substantial advances in the biology of human embryonic stem (ES) cells, and the technology for working with them, have been made over the past 10 years. Regulatory frameworks for their study are well developed, although some countries remain particularly restrictive. Markers and criteria for characterising human ES cells are also generally agreed, and protocols for promoting their differentiation are being established, providing the groundwork for the development of applications over the next 10 years. The recent appearance of technology to convert somatic cells to ‘induced Pluripotent Stem Cells’ closely resembling ES cells will certainly speed up these developments.
Similar content being viewed by others
Main
This October marks the 10th anniversary of the first reported derivation of human embryonic stem (ES) cells.1 The report was met with much acclaim as it was quickly recognized that the ability of these pluripotent stem cells to differentiate into all cell types of the human body could provide a source of cells for replacing tissues lost to injury and disease—the ultimate goal of regenerative medicine. Inevitably, these early discussions overlooked the substantial hurdles that lay in the way, leading to disappointment in some quarters that, 10 years later, more progress has not been made. Yet in the more mundane world of small molecule drugs, the pharmaceutical industry typically expects a delay of 10–15 years between initial discovery and delivery to market, whereas in the perhaps more pertinent case of monoclonal antibodies, first reported in 1975, it is only in the past few years, 30 years on, that significant monoclonal antibody-based treatments for cancer have appeared on the market.
The great advantage of ES cells over other stem cells is that they can generate many potentially useful cell types—but that is also their disadvantage. To use ES cells effectively in regenerative medicine, or in other applications, such as disease modelling or drug discovery, applications that are often over looked in popular discussion, it is essential that we understand how to control their differentiation. This is necessary if one is to expand cultures of the undifferentiated cells to a usable scale, free of potential pathogens. It is also necessary if one is to produce specified cell types, free of other unwanted cells, and if the genetic fidelity of the cells is to be preserved, a factor that was not immediately apparent when these cells were first derived. These issues present substantial challenges, but slowly they are being overcome as we begin to gain insights into the biology of these fascinating cells.
DERIVATION AND MAINTENANCE OF HUMAN ES CELLS
Human ES cells are derived by disrupting early human embryos at the blastocyst stage and placing them in culture under specialized conditions.1 At this time, it is variously estimated that over 300 such lines have been derived in many countries around the world. However, the need to destroy embryos raised many ethical and legal issues that have been addressed in different ways in different countries. The UK has been at the forefront of regulation in this area and a 1990 law specifically established the legal framework for experimental work with human embryos. This law provided for a body, the Human Fertilisation and Embryo Authority, from which any work, whether for assisted conception or research to produce ES cells, must be licensed. A separate body, the UK Stem Cell Steering Committee oversees subsequent work with human ES cell lines once established. Other countries took a wide variety of different approaches. Some completely banned work with human ES cells, others permitted work with existing ES cells and yet others provided no rules whatsoever. In the USA, although Federal funds can be used only for research on a small number of lines established before 9 August 2001, unregulated research in most States is legal if funded privately. This patchwork of national rules has been changing significantly over the years and a summary of the current position can be found on the Website of the Ethics Working Party of the International Stem Cell Forum (http://www.stemcellforum.org/forum_initiatives/ethics_working_party.cfm).
A significant practical problem has been the conditions used for human ES derivation and growth. Lines have generally been derived on feeder layers of inactivated mouse embryo fibroblasts, in a medium containing fetal bovine serum (FBS). Quite quickly FBS was replaced by a propriety commercial preparation, Knock-out Serum Replacement supplemented with basic fibroblast growth factor (FGF-2). However, the presence of animal-derived components is considered a problem for eventual clinical applications. Considerable work has been devoted to switching first to human fibroblast feeders and then to chemically defined media.2 At the same time, various groups have established facilities to derive lines under Good Manufacturing Practice (GMP) conditions that fully document the provenance of the lines derived. A few lines derived to GMP standards have been reported,3 but the regulatory requirements for clinical trials with human patients remain uncertain.
Another problem is the realization that human ES cells may acquire genetic and epigenetic changes on prolonged passage.4 Interestingly, some of the karyotypic changes seen match those seen in embryonal carcinoma (EC) cells, the malignant counterparts of ES cells,5 suggesting that similar selection pressures for cells that have an altered balance of a propensity for differentiation, apoptosis and self-renewal in favour of self-renewal may operate in both situations. There has been considerable discussion as to the causes of such genetic changes, which we have termed ‘culture adaptation’, and some have suggested that particular culture conditions or passaging methods may favour the acquisition of such changes, but these issues remain to be elucidated. Resolving the causes for the acquisition of genetic variation is a crucial element in developing methods for scaling up cells for particular applications.
CHARACTERIZATION
The derivation of human ES cells (Figure 1) was preceded by studies of mouse ES cells and human EC cells, the malignant counterparts of ES cells.6 These studies already pointed to significant differences between human and mouse ES cells. Thus, human EC cells are typically capable of trophectodermal differentiation, a characteristic not seen in mouse ES cells. Also among a series surface marker antigens, stage specific embryonic antigen-1 (SSEA1), a well-established marker of mouse ES cells, is not expressed by human EC cells. Likewise, other glycolipid antigens (SSEA3 and -4) in this series are expressed by human EC cells (Figure 1), but not by mouse EC and ES cells. Yet other differences included the expression of MHC class one antigens, Thy1 and the high molecular weight glycoprotein antigens TRA-1-60 and TRA-1-81 by human but not mouse cells. Interestingly, the patterns of marker antigen expression by human ES cells are not uniform (Figure 1) and the possibility of subsets of cells with different functional capacities within the stem cell compartment has been suggested.7 Once human ES cells were available, the differences in expression patterns of these antigens between mouse and human ES cells were confirmed.8 Further, the similarity of the surface antigen phenotype of the ES cells of both species to that of cells of the inner cell masses of the embryos of the respective species was also confirmed.9
The phenotype of human ES cells. (a) Phase-contrast photomicrograph of an undifferentiated human ES cell colony showing the typical morphology of these cells; note the high nuclear to cytosolic ratio typical of undifferentiated ES cells. (b, c): Human ES cells immunostained for expression of the marker antigens SSEA3 (b) and TRA-1-60 (c); note that SSEA3 marks many but not all of the cells, whereas TRA-1-60 marks almost all, suggesting the existence of heterogeneity and subsets of cells within the stem cell compartment. (d) Neurons derived by differentiation from human ES cells, immunostained for β-III-tubulin.
Nevertheless, there are also substantial similarities. Human and mouse ES cells all express the transcription factors NANOG, OCT4 and SOX2, and high levels of alkaline phosphatase.8 Functional studies further suggested similar roles of these transcription factors in maintaining the pluripotent stem cell state. For example, reduced expression of OCT4 in human ES cells by genetic manipulation results in trophectodermal differentiation.10 Analysis of the potential genomic interaction sites of the core transcriptional machinery of SOX2, OCT4 and NANOG11 has started to reveal the possible transcriptional core circuitry that exists in the undifferentiated stem cells.
However, despite the similarities of the intrinsic control of mouse and human ES cells by these transcription factors, the effects of extrinsic factors are substantially different. Thus, although mouse ES cells can be maintained without feeder cells in the presence of the cytokine LIF, this has no effect on human ES cells. On the other hand, human ES cells respond to high levels of FGF, whereas mouse ES cells do not. Further, activin and TGFβ support the self-renewal of human ES cells, whereas BMPs induce their differentiation and promote self-renewal of mouse ES cells.12
A possible explanation for these differences is the suggestion that human ES cells correspond to cells of the epiblast rather than the inner cell mass as is proposed for the mouse. In support of this, pluripotent stem cells with properties similar to human ES cells have been derived from later stage mouse and rat embryos at about the time of gastrulation.13, 14 However, what is not explained by these observations is that both human and mouse ES cells are derived by explantation of cells from the same corresponding embryonic stage, the blastocyst.
DIFFERENTIATION
The differentiation of human ES cells is readily induced by culture as aggregates, either in suspension as ‘embryoid bodies’ or by leaving monolayer cultures to overgrow, yielding cultures that contain multiple cell types (Figure 1). From these, it may be possible to isolate specific cells that could be useful in subsequent applications. Among such cells are neural lineage cells, and a variety of procedures have been developed to permit isolation of neural stem cells and various neural cell types from such populations.15 Other cell types may also be isolated in varying degrees of purity, including pancreatic-β-like cells,16 cardiomyocytes17 and hepatocyte-like cells.18 Although these procedures provide a starting point for producing specific cells for possible applications, whether in regenerative medicine, or as tools for drug discovery or as disease models, little is understood about the underlying mechanisms. Although some limited success has been achieved by manipulating culture conditions to drive differentiation of human ES cells along particular lineages, there is little evidence to support the view that such conditions specifically direct differentiation rather than select for propagation or survival of cells generated by spontaneous processes. A number of chemical agents and growth factors such as retinoic acid or BMP are well known to have physiological roles in embryonic development and have also been used to promote differentiation of ES cells in culture.19 However, even in these cases, although there is a tendency for differentiation to follow broad lineages such as ectodermal or mesodermal, the cell types generated also tend to be heterogeneous.
CONCLUSION
In the 10 years since their first derivation, human pluripotent ES cells have been transformed from cells requiring specialized expertise for culture and analysis to much more accessible items in the cell biologist's toolbox. Their potential for making available scalable and reproducible sources of human cells for drug and toxicology screening is evident, and the pharmaceutical industry is moving towards exploiting these opportunities, for example evidenced by the formation of Stem Cells for Safer Medicines (www.sc4sm.org), a public–private partnership sponsored by the UK Government and several large pharmaceutical companies. Although major hurdles remain in understanding the basic biology of these cells, the substantial progress made in the past 10 years has laid the foundations for development of applications in regenerative medicine over the next 10 years. It remains to be seen when and where the first applications will be made, but clinical trials for treatments for age-related macular degeneration, spinal cord injury and neurological diseases such as Parkinson's disease are likely possibilities.
A recent result that promises to revolutionize the field is the remarkable discovery that just four transcription factors are able to elicit the re-programming of fully differentiated cells to an ES-like state - so called induced pluripotent stem (iPS) cells.20 These cells have opened up the possibility of ‘fixing’ a particular genotype (either normal or diseased) in pluripotent stem cells and allowing serious attempts at developing robust in vitro disease models. Although the precise relationship of iPS and ES cells remains to be explored in detail, the advent of iPS cell technology circumvents the ethical, legal and logistical problems associated with the need for human embryos to derive ES cell lines, and has started a democratization process that should see a substantial expansion in the study of pluripotent cell biology. The availability of human versions of this class of cells has re-invigorated the study of how cells in higher multi-cellular organisms decide their fate. Thus, the next 10 years promises exciting advances in the understanding of the mechanisms that allow cells to adopt particular fates, with all that implies for human developmental biology, cancer research and regenerative medicine.
References
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147.
Ludwig TE, Bergendahl V, Levenstein ME, et al. Feeder-independent culture of human embryonic stem cells. Nat Methods 2006;3:637–646.
Crook JM, Peura TT, Kravets L, et al. The generation of six clinical-grade human embryonic stem cell lines. Cell Stem Cell 2007;1:490–494.
Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 2004;22:53–54.
Harrison NJ, Baker D, Andrews PW . Culture adaptation of embryonic stem cells echoes germ cell malignancy. Int J Androl 2007;30:275–281; discussion 281.
Andrews PW . From teratocarcinomas to embryonic stem cells. Philos Trans R Soc Lond B Biol Sci 2002;357:405–417.
Enver T, Soneji S, Joshi C, et al. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum Mol Genet 2005;14:3129–3140.
Adewumi O, Aflatoonian B, Ahrlund-Richter L, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol 2007;25:803–816.
Henderson JK, Draper JS, Baillie HS, et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells 2002;20:329–337.
Matin MM, Walsh JR, Gokhale PJ, et al. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells 2004;22:659–668.
Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005;122:947–956.
Avery S, Inniss K, Moore H . The regulation of self-renewal in human embryonic stem cells. Stem Cells Dev 2006;15:729–740.
Brons IG, Smithers LE, Trotter MW, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007;448: 191–195.
Tesar PJ, Chenoweth JG, Brook FA, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007;448:196–199.
Zhang SC, Wernig M, Duncan ID, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001;19:1129–1133.
Liew CG, Shah NN, Briston SJ, et al. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS ONE 2008;3:e1783.
Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 2001;108:407–414.
Hay DC, Zhao D, Fletcher J, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 2008;26:894–902.
Schuldiner M, Yanuka O, Itskovitz-Eldor J, et al. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 2000;97:11307–11312.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872.
Acknowledgements
This study was supported, in part, by grants from the MRC, BBSRC and from ESTOOLS, a European Union 6th Framework Integrated Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gokhale, P., Andrews, P. Human embryonic stem cells: 10 years on. Lab Invest 89, 259–262 (2009). https://doi.org/10.1038/labinvest.2008.162
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2008.162
Keywords
This article is cited by
-
Generation of pluripotent stem cells without the use of genetic material
Laboratory Investigation (2015)