Abstract
Hydrogen sulfide (H2S) has been traditionally known for its toxic effects on living organisms. The role of H2S in the homeostatic regulation of pancreatic insulin metabolism has been unclear. The present study is aimed at elucidating the effect of endogenously produced H2S on pancreatic insulin release and its role in diabetes development. Diabetes development in Zucker diabetic fatty (ZDF) rats was evaluated in comparison with Zucker fatty (ZF) and Zucker lean (ZL) rats. Pancreatic H2S production and insulin release were also assayed. It was found that H2S was generated in rat pancreas islets, catalyzed predominantly by cystathionine γ-lyase (CSE). Pancreatic CSE expression and H2S production were greater in ZDF rats than in ZF or ZL rats. ZDF rats exhibited reduced serum insulin level, hyperglycemia, and insulin resistance. Inhibition of pancreatic H2S production in ZDF rats by intraperitoneal injection of DL-propargylglycine (PPG) for 4 weeks increased serum insulin level, lowered hyperglycemia, and reduced hemoglobin A1c level (P<0.05). Although in ZF rats it also reduced pancreatic H2S production and serum H2S level, PPG treatment did not alter serum insulin and glucose level. Finally, H2S significantly increased KATP channel activity in freshly isolated rat pancreatic β-cells. It appears that insulin release is impaired in ZDF because of abnormally high pancreatic production of H2S. New therapeutic approach for diabetes management can be devised based on our observation by inhibiting endogenous H2S production from pancreas.
Similar content being viewed by others
Main
Known as a swamp gas or ‘rotten egg’ gas, hydrogen sulfide (H2S) has yielded a public image of air pollutant for centuries. Physiological importance of H2S as a gasotransmitter has been realized for less than a decade. Endogenous production of H2S from L-cysteine is catalyzed by cystathionine β-synthase (CBS) and/or cystathionine γ-lyase (CSE) with ammonium and pyruvate as co-products.1 This process occurs in different organs and tissues, such as neuronal, vascular, and intestinal tissues.2 Physiological concentrations of circulating H2S have been reported in the range of 45–300 μM.1 At this physiological range, exogenous H2S has been shown to relax different vascular tissues, including isolated rat aortae and perfused mesenteric artery bed.3, 4, 5 Altered cell proliferation or apoptosis induced by H2S has also been widely reported.6, 7, 8, 9, 10
Endogenous production and physiological function of H2S in pancreas have been studied with identification of both CBS and CSE in rat pancreatic tissues or cloned rat pancreatic β-cell line.11, 12 In recent years, pathophysiological implications of the CSE/H2S system in diabetes have been reported.12 CSE mRNA expression and H2S formation in rat pancreas was significantly increased after diabetes induction by streptozotocin injection.12 CBS expression was reported in pancreatic acinar cells.13 Yusuf et al12 also detected the expression of CBS mRNA in mouse pancreas but whether this expression was located to pancreatic islets or other parts of the pancreas was not clear. The same research team later examined the expression of CBS in HIT-T15 cells, an insulinoma pancreatic β-cell line derived from Syrian hamster,14 and no CBS protein was shown in these cells. In contrast, Kaneko et al15 reported the expression of CBS protein in mouse pancreatic islets and a mouse β-cell line MIN6. We have shown that CSE is the major H2S-generating enzyme in pancreatic islet, demonstrated by significant CSE protein expression,11 and by our published data that PPG abolished the most H2S production from cultured INS-1E cells.11
Among demonstrated cellular and molecular mechanisms for physiological effects of H2S are the activation of KATP channels by H2S. In vascular smooth muscle cells, exogenous and endogenous H2S activated KATP channels at whole-cell and single channel levels.16, 17 It is reasoned that H2S would also affect other excitable cells that have functional KATP channels. Pancreatic β-cells are among these cells and our earlier study in fact demonstrated the activation of KATP channels in an insulin-secreting cell line, INS-1E, by H2S.11 The KATP channel-dependent effect of H2S on insulin release has also been shown in another insulin-secreting cell line, HIT-T15.14 Insulin release from pancreatic islets is a critical event in homeostatic control of glucose metabolism and in pathogenic process of insulin resistance development, including diabetes. As opening of KATP channels leads to membrane hyperpolarization, insulin release from pancreatic islets would be inhibited because of reduced Ca2+ influx.
Given that altered insulin release is critical for the development of diabetes and this process is likely to be modulated by H2S, it became imperative to gain better understanding on the role of H2S metabolism in the pathogenesis of diabetes and the underlying mechanisms. The present study focused on the altered endogenous pancreatic H2S production in Zucker diabetic fatty rats, a genetic animal model of type 2 diabetes mellitus. We also attempted to correlate this altered H2S production with insulin release from pancreatic islets and with the development of diabetes. Whether lowering endogenous pancreatic H2S levels of diabetic animals could reverse diabetes development was further investigated. Our results suggest that insulin release is impaired in diabetic animals and that inhibition of abnormally increased endogenous pancreatic H2S production in diabetes might represent a novel avenue for diabetes treatment.
MATERIALS AND METHODS
Animal Model of Diabetes and Isolation of Pancreatic Islet and β-Cells
Male Zucker diabetic fatty (ZDF) rats, Zucker fatty (ZF) rats, and Zucker lean (ZL) rats (6- to 18-week-old) were received from Charles River Laboratories Inc. (Wilmington, MA). These animals were housed at 21°C with 12-h light/dark cycles. After anesthetization of rats by intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight), pancreatic islets were digested with collagenase and hand picked under microscope. The isolated islets (800–1000) were then dispersed into single cells by a brief (3–4 min) incubation in Ca2+-free medium consisting of (in mM) 120 NaCl, 5 KCl, 2 MgSO4, 4 glucose, 1 EGTA, 25 Hepes-NaOH (pH 7.4), and 1% (w/v) bovine serum albumin (BSA). These cells were later used either for the patch-clamp recording or H2S production assay. The protocol for animal experiment was approved by the University of Saskatchewan Committee on Animal Care and Supply.
Measurements of Pancreatic islet H2S Production and Insulin Level
The measurement of H2S production rate in isolated pancreatic islets followed the established protocol in our laboratory.3, 4, 5, 16 The isolated islets were collected by centrifuging at 1000 r.p.m. for 3 min and then homogenized in 50 mM ice-cold potassium phosphate buffer (pH 6.8). Homogenate (10%, w/v) was added to the reaction mixture containing (mM): 100 potassium phosphate buffer (pH 7.4), 10 L-cysteine, and 2 pyridoxal 5′-phosphate. Cryovial test tubes (2 ml) were used as the center wells, each containing 0.5 ml 1% zinc acetate as trapping solution and a filter paper 2 × 2.5 cm2 to increase air: liquid-contacting surface. Reaction was performed in a 25 ml Erlenmeyer flask (Pyrex, USA). The flasks containing the reaction mixture and center wells were flushed with N2 before being sealed with a double layer of Parafilm. Reaction was initiated by transferring the flasks from ice to a 37°C shaking water bath. After incubating at 37°C for 90 min, 0.5 ml of 50% trichloroacetic acid was added to stop the reaction. The flasks were sealed again and incubated at 37°C for another 60 min to ensure a complete trapping of H2S released from the mixture. Contents of the center wells were then transferred to test tubes, each containing 3.5 ml of water. Subsequently, 0.5 ml of 20 mM N,N-dimethyl-p-phenylenediamine sulfate in 7.2 N HCl was added immediately followed by addition of 0.5 ml 30 mM FeCl3 in 1.2 N HCl. Absorbance of the resulting solution at 670 nm was measured 20 min later with a spectrophotometer. H2S content was calculated against the calibration curve of standard H2S solutions. Serum H2S levels were measured on duplicated samples with a sulfide electrode (Lazar Research Laboratories, Los Angeles, CA)18 on an Accumet Model 10 pH meter (Fisher Scientific, Pittsburgh, PA).
For insulin release determination, the isolated pancreatic islets were washed and preincubated in glucose-free Krebs–Ringer-bicarbonate medium (pH 7.4) containing (mM): 135 NaCl, 3.6 KCl, 5 NaHCO3, 0.5 NaH2PO4, 0.5 MgCl2, 1.5 CaCl2, 10 Hepes, and 0.1% BSA. After 60 min preincubation, islets were incubated at 37°C in the presence of different glucose concentrations with or without PPG. At the end of 30-min incubation period, the incubation medium was collected and insulin content determined using the rat insulin ELISA kit (Mercodia AB, Sylveniusgatan, Uppsala, Sweden).
Samples of blood were collected into EDTA-coated tubes from overnight fasted rats. The blood samples were then centrifuged (2500 g) at 4°C for 15 min and derived serum stored at −20°C. Insulin levels in serum were determined also with a rat-specific Insulin ELISA kit (Mercodia AB).19, 20, 21 Blood hemoglobin A1c (HbA1c) level was measured by the Biochemistry Laboratory of Royal Hospital of the University of Saskatchewan.
Real-Time Quantitative PCR
Real-time PCR was performed on an iCycler iQ real-time PCR detection system using SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA). Negative controls for this experiment were samples without a template. For quantification, the target gene was normalized to the internal standard gene β-actin. The primers of CSE (GenBank no. AY032875) were 5′-AGCGATCACACCACAGACCAAG-3′ (sense, position 432–453) and 5′-ATCAGCACCCAGAGCCAAAGG-3′ (antisense, position 589–609). These primers produced a product of 178 bp. The primers of CBS (GenBank no. NM012522) were 5′-CAACACAGGGATCGGGCT-3′ (sense, position 580–597) and 5′-AGGACGGTGGGGATGAAG-3′ (antisense, position 1063–1080). These primers produced a product of 501 bp. The primers of β-actin (Ambion) produced a product of 295 bp. A standard curve was constructed with a series of dilutions of total RNA (Austin, TX, USA) transcribed to cDNA using the protocol outlined above to confirm the same amplifying efficiency in the PCR. A standard melting curve analysis was performed using a thermal cycling profile that began at 95°C for 1 min, decreased to 55°C for 1 min, and then ramped to 95°C in one-degree increments to confirm the absence of primer dimers. Product size was determined by running PCR products on a 1.8% agarose gel. Relative mRNA quantification was calculated by using the arithmetic formula ‘ ’, where ΔCT is the difference between the threshold cycle of a given target cDNA and an endogenous reference cDNA.9, 22, 23 Thus, this value yields the amount of the target normalized to the endogenous reference of β-actin.
’, where ΔCT is the difference between the threshold cycle of a given target cDNA and an endogenous reference cDNA.9, 22, 23 Thus, this value yields the amount of the target normalized to the endogenous reference of β-actin.
Western Immunoblotting
Isolated rat pancreatic islets were homogenized using a polytron homogenizer. The supernatants containing crude cellular proteins were resolved on a 10% SDS-PAGE gel, and transferred onto the PVDC nitrocellulose membrane.24 The membrane was blocked with 3% nonfat dry milk solution in PBS at room temperature for 2 h and rinsed three times with PBS before incubating firstly with primary antibody (1:500 for CSE antibody, 1:5000 for β-actin antibody) and then with the HRP-conjugated secondary antibody (1:5000). The immunoreactions were visualized by ECL and exposed to X-ray film (Kodak Scientific Imaging film, X-omat Blue XB-1).
To prepare the anti-CSE polyclonal antibody, synthesized, and conjugated targeted peptide VGLEDEQDLLEDLD (Genbank accession no. AY083352, 377–392) were used to immunize male New Zealand rabbits. After the titer of anti-CSE antisera reached 1:4000, they were subjected to purification by protein G affinity. Purified antibody was eluted with 0.1 M glycine buffer (pH 2.5), and stored at −80°C for later use.
Glucose Tolerance Test and Insulin Tolerance Test
Glucose tolerance test (GTT) was conducted by injecting the rats, after 12-h fast, with 1.5 g dextrose per kg body weight in a 50% dextrose solution intraperitoneally. Blood samples were collected from tails at different times after dextrose injection. Glucose levels were monitored using blood glucose strips (Onetouch, LifeScan). For insulin tolerance test (ITT), the rats were fasted for 12 h and then received insulin injection (1 U/kg, i.p.). Blood glucose levels were assessed before and 15, 30, 45, 60, and 90 min into the ITTs.25
Electrophysiological Recording of KATP Channel Currents
Suspensions of dispersed cells from pancreatic islets were centrifuged at 100 r.p.m. for 3 min and the collected cells were then resuspended in Hepes-buffered RPMI 1640 (Sigma, USA). The freshly isolated single cells were tested for their KATP channel response to high glucose to confirm their identity as pancreatic β-cells. Only those cells of which KATP channel activity was significantly decreased by high glucose (16 mM) and inhibited by KATP channel blocker, gliclazide, were used in this study.11 The cells in the recording chamber were superfused continuously with the bath solution at a rate of about 2 ml/min. A complete solution change in the recording chamber was accomplished within 30 s. All electrophysiological experiments were conducted at room temperature (20–22°C).
The whole-cell KATP channel currents of single β-cells were recorded at a membrane potential of −60 mV.17 Recording electrodes were pulled from soft microhematocrit capillary tubes (Fisher Scientific, Nepean, ON) with tip resistance of 2–4 MΩ when filled with the pipette solution. Currents were recorded with an Axopatch 200-B amplifier (Axon Instruments), controlled by a Digidata 1200 interface and pCLAMP software (version 6.0, Axon Instruments). Membrane currents were filtered at 1 kHz with a four-pole Bessel filter, digitized, and stored. At the beginning of each experiment, junction potential between pipette and bath solutions was electronically adjusted to zero. The bath solution for recording the whole-cell KATP currents contained (in mM) NaCl 140, KCl 5.4, MgCl2 1.2, HEPES 10, EGTA 1, glucose 5 (pH adjusted to 7.4 with NaOH). The pipette solution was composed of (in mM) KCl 140, MgCl2 1, EGTA 10, HEPES 10, glucose 5, Na2ATP 0.3, MgGDP 0.5, and PPG 5 (pH adjusted to 7.2 with KOH). The K+ concentration of bath solutions was increased to 140 mM, after stable KATP currents were recorded, by the removal of equal-molar NaCl.
Chemicals and Statistical Analysis
The H2S-saturated solution (0.09 M) was freshly made by bubbling pure H2S gas (Praxair; Mississauga, Canada) into Krebs' solution at 30°C for 40 min as described previously.5 H2S stock solution was diluted to different concentrations into cell culture medium, and the pH of medium was adjusted to 7.4. Data are expressed as mean±s.e.m. Multiple comparisons were made with one-way ANOVA followed by a post hoc analysis (Tukey's test). Statistical significance was set at P<0.05.
RESULTS
Pancreatic Production of H2S
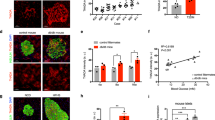
To elucidate enzymatic basis of pancreatic H2S production, we firstly examined transcriptional expression of CSE and CBS. Although both CSE and CBS transcripts were detected in pancreatic islet tissues, quantitative comparison using real-time RT-PCR revealed that expression level of CSE was significantly higher than that of CBS in 16-week-old diabetic ZDF or nondiabetic ZF rats (Figure 1a). As CBS mRNA was extremely weak in pancreatic islet tissues, the following western blot studies were focused on CSE proteins. The expression level of CSE proteins was significantly higher in pancreatic islets of 16-week ZDF rats than that of age-matched ZL and ZF rats (Figure 1b). Production rate of endogenous H2S in pancreatic islet tissues was also significantly greater in ZDF than that of ZF and ZL rats (Figure 1c). Neither CSE protein expression nor H2S production in pancreatic islets was different between nondiabetic ZL and ZF rats.
Pancreatic islet expression of H2S-generating enzymes and production of H2S in 16-week rats. (a) Expression of CBS and CSE mRNA. *P<0.05 ZF rats and #P<0.01 vs CBS from the same types of tissues. n=5 for each group. (b) Expression of CSE proteins. *P<0.05 vs all other groups; n=4 for each group. (c) H2S production rate. *P<0.05 vs PPG-treated group; #P<0.05 vs ZL or ZF rats; n=3–5 for each group.
Diabetic Status of ZDF Rats
At age of 8 weeks, ZDF rats did not develop diabetes and their overnight fasted blood glycated haemoglobin (HbA1c) level was 3.68±0.31% (n=5, P>0.05 vs ZL rats). However, even at this prediabetic stage, increased expression of CSE protein in pancreatic islets from ZDF rats already became significant (Figure 2).
Blood glucose level of both ZL and ZF rats at different ages remained within normal range (Figure 3a and b). Hyperglycemia gradually developed, however, in ZDF rats as they aged and reached plateau at 12–13 weeks (Figure 3c). HbA1c level was significantly higher in 16-week ZDF (6.34±0.27%, n=6) than in age-matched ZL (4.63±0.10%, n=4) and ZF rats (5.4±0.4%, n=5; P<0.05).
Decreased hyperglycemia in ZDF rats by lowering endogenous H2S production. PPG treatment started at the age of 12 weeks. (a) Blood glucose level in ZL rats (n=8). (b) Blood glucose level in ZF rats with or without PPG treatment (33.9 mg/kg) for 4 weeks; n=4 for each group. (c) Hyperglycemia in ZDF rats and its reduction by PPG treatment (33.9 mg/kg) for 4 weeks; n=4 for each group. *P<0.05 vs sham-treated ZDF.
Correlation of H2S Production and Glucose Metabolism in Diabetes
To reveal the role of endogenously produced H2S in the development of diabetes, 12-week ZDF or ZF rats were treated with PPG (33.9 mg/kg/day, i.p.) for 4 weeks or with AOA (30–100 mg/kg per day, i.p.) for 6 weeks to specifically inhibit CSE activity or CBS activity, respectively. Control groups of ZDF and ZF rats were injected with equal volumes of PBS, the solvent for PPG or AOA, for the same period. For each injection, the rats received about 0.45–0.65 ml drug solution or PBS dependent on their body weights. At the end of 4- or 6-week injection period, the animals (aged 16–18 weeks) were terminated and assayed.
PPG treatment drastically reduced H2S production rate to near zero in pancreatic islets from both ZDF and ZF rats (Figure 1c). Although it had no effect on blood glucose level of ZF rats aged 8–16 weeks (Figure 3b), the 4-week PPG treatment brought hyperglycemia of ZDF rats down to near normal level (Figure 3c). HbA1c level of ZDF rats (6.3±0.3%, n=5) was significantly lowered by PPG treatment to 5.3±0.04% (n=3; P<0.05), which was still higher than that of ZL rats (P<0.05) but not significantly different from that of ZF rats (n=5).
Although CBS expression was minimal in pancreatic islet tissues (Figure 1a), the role of CBS in the development of hyperglycemia of ZDF was nevertheless investigated. Treatment with AOA, a specific blocker of CBS, for 6 weeks did not significantly change hyperglycemic status of ZDF rats. HbA1c level of ZDF rats remained as high as 7.37±0.02 or 7.14±0.03% after AOA treatments at 30 mg/kg per day (n=4) or 100 mg/kg per day (n=4), respectively, whereas it was 6.3±0.3% in the age-matched and untreated ZDF rats (n=5; P>0.05).
Reduced hyperglycemia and HbA1c levels in PPG-treated ZDF could result from improved insulin release, which would facilitate insulin-sensitive glucose metabolism. After glucose injection, blood glucose levels of nondiabetic control ZL or ZF rats initially arose but then quickly fell to the preinjection levels within 2 h (Figure 4a). ZDF rats have a significant deficiency in glucose clearance. Elevation of blood glucose level of ZDF rats after glucose injection followed a similar time course as nondiabetic rats. However, the blood glucose level of ZDF rats remained elevated throughout the entire study period. In PPG-treated ZDF rats, elevated blood glucose level significantly declined during the 2 h post-glucose injection period, albeit not completely back to the preinjection level (Figure 4a). There was no significant difference in blood glucose levels during GTT between control ZL rats and PPG-treated ZL rats (Figure 4a).
Glucose tolerance of 16-week-old Zucker rats. ZDF or ZL rats were treated with PPG for 4 weeks. (a) Changes in blood glucose levels during glucose tolerance test (GTT). (b) Changes in serum insulin levels during GTT. *P<0.05 vs all other groups; #P<0.05 vs ZL or ZF; n=5 for ZL, ZF, and ZDF; n=6 for ZDF+PPG and ZL+PPG.
During GTT, the increase in serum insulin level of ZDF rats was delayed with much lower amplitude as compared with that of ZL rats (Figure 4b). PPG treatment elevated the basal level of serum insulin of ZDF rats. Both the rate of increase and the peak amplitude of serum insulin level of PPG-treated ZDF rats were significantly increased in comparison with that of untreated ZDF rats (Figure 4b). Moreover, PPG treatment had no significant effect on serum insulin level of ZL rats (Figure 4b). Once reaching their peaks, serum insulin levels of all groups of rats returned to their respective basal levels within 1 h.
Alterations in Serum Insulin Level
Serum insulin levels in all groups of 8-week rats were significantly lower than those of 16-week-old rats. Prediabetic 8-week ZDF rats had a serum insulin level of 1.34±0.08 ng/ml (n=5), being significantly higher than that of age-matched ZL (0.78±0.01 ng/ml, n=3) and ZF rats (0.96±0.20 ng/ml, n=5; P<0.05). Serum insulin level was significantly lower in 16-week ZDF rats than age-matched nondiabetic ZL and ZF rats (P<0.01), although it was significantly higher in ZF than in ZL rats (P<0.01; Figure 5a). Correlated with low serum insulin level was the elevated serum H2S level in ZDF rats. There was no significant difference in serum H2S level between ZF and ZL rats (P>0.05), but a significantly higher serum H2S level was detected in ZDF rats than in ZF and ZL rats (P<0.05; Figure 5b). PPG treatment increased serum insulin of ZDF rats to a level comparable to that of ZL rats (P>0.05), but had no effect on that of ZF or ZL rats (Figure 5a).
Insulin Tolerance and Pancreatic Insulin Release
Although they had a significantly lower serum insulin level (Figure 5a), ZDF rats still developed insulin resistance (Figure 6a). PPG treatment did not improve the impaired insulin resistance in ZDF rats (Figure 6a). With a glucose challenge at basal level (5 mM), insulin release was significantly greater from ZF islets than ZL and ZDF islets. Although insulin release from ZF islets in response to a high-glucose challenge (20 mM) was still greater than other groups, insulin release from ZDF islets was significantly lower than that from all other groups. PPG (5 mM) incubation of ZDF islets largely restored the suppressed insulin release (Figure 6b).
Insulin sensitivity of and glucose-induced insulin release from 16-week-Zucker rats. (a) Insulin tolerance tests (ITTs) in different groups of rats; n=4–6 for each group. (b) Insulin release from the isolated pancreatic islets in vitro in response to glucose stimulations. *P<0.05 vs all other groups exposed to the same glucose concentration; n=4 for each group.
Opening of Pancreatic KATP Channels by H2S
Albeit major efforts have been focused on altered insulin levels in diabetes, not much is known on the underlying mechanisms toward impaired pancreatic release of that hormone. In freshly isolated rat pancreatic β-cells, shifting K+ concentration of the bath solution from 5.4 to 140 mM significantly increase the inward KATP channel currents (Figure 7a and b). With a holding potential of −60 mM and in the absence of Ca2+ from the bath solution, neither voltage-dependent K+ channel nor calcium-activated K+ channels would have been activated.26, 27 H2S at 100 μM significantly increased KATP channel currents that were subsequently inhibited by gliclazide (1 μM), a classical KATP channel blocker,28, 29 in β-cells from both ZL (Figure 7a) and ZDF rats (Figure 7b). The amplitude of KATP channel currents at resting condition appeared to be lower in ZDF β-cells than ZL β-cells (Figure 7c).
Effect of H2S on KATP channel currents in pancreatic β-cells freshly isolated from ZL and ZDF rats. (a) Effect of H2S (100 μM) and gliclazide (1 μM) on KATP channel currents in β-cells from ZL rats. (b) Effect of H2S (100 μM) and gliclazide (1 μM) on KATP channel currents in β-cells from ZDF rats. (c) Summary of the effect of H2S on KATP channel currents in different types of β-cells. *P<0.01, n=4 for each group. Membrane potential was at −60 mV. The broken lines in (a and b) denote zero current level.
DISCUSSION
Altered insulin metabolism and reduced insulin sensitivity are two trademarks of diabetes mellitus. Albeit with concentrated attention being paid to different circulating insulin levels in diabetes, not much so has been known about altered insulin release from pancreas in diabetes and the underlying mechanisms. Insulin release from pancreatic β-cells is tightly coupled to glucose metabolism. In addition to an amplifying pathway putatively involving mitochondrial production of glutamate,29 an extracellular calcium entry-dependent mechanism controlled by KATP channels in β-cell membrane is a pivotal event in glucose-regulated insulin release cascade. In this event, increase in circulating glucose results in production of ATP from β-cell mitochondria. A high ATP/ADP ratio subsequently closes KATP channels in pancreatic β-cells, leading to membrane depolarization.28 Resultant opening of voltage-dependent calcium channels injects more calcium into cytosolic milieu. This eventually leads to exocytosis of insulin granules.
In addition to affecting ATP levels, H2S also regulates KATP channel activities. Recently, we showed that in INS-1E cells exogenous H2S at 100 μM had no effect on the whole-cell KATP currents. After concentration of H2S was elevated to 300 μM, the amplitude of KATP current in INS-1E cells was increased.11 PPG is a specific inhibitor of CSE.3, 17 Interestingly, in the presence of PPG that inhibited endogenous H2S production or with high glucose to inhibit KATP channel activities in INS-1E cells, exogenous H2S at concentrations equal to or lower than 100 μM significantly increased KATP channel currents. These results suggest that the functions of KATP channels in INS-1E cells are related to endogenous H2S metabolism. After knocking down CSE gene expression in INS-1E cells, endogenous H2S level was significantly lowered and the whole-cell KATP channel current density was significantly reduced in INS-1E cells in comparison with wild-type native cells,11 further demonstrating the stimulatory effect of endogenous H2S on KATP channels. INS-1E cells are from a pancreatic insulinoma cell line. Further evidence has been provided that 100 μM H2S directly reduced insulin secretion from INS-1E cells.11
The aforementioned studies have not addressed the relationship between pancreatic H2S metabolism and insulin release and development of hyperglycemia in diabetes. If endogenous H2S level was elevated in pancreatic β-cells, insulin release would be reduced and hyperglycemia would occur. The first step in proving this hypothesis is to determine endogenous production of H2S in pancreatic islet and its molecular basis. In the present study, we detected significant pancreatic islet production of H2S. In comparison with the expression level of CSE, the expression level of CBS mRNA in rat pancreatic islets is extremely low (Figure 1a). Therefore, it is concluded that pancreatic islet H2S production is predominantly regulated by CSE, rather than CBS.
Next, we tried to elucidate the correlation of pancreatic H2S production with diabetes development by using ZDF rats. These rats represent a genetic animal model of type 2 diabetes mellitus, characterized with hyperglycemia, and peripheral insulin resistance. Serum insulin level of ZDF rats is initially higher but significantly drops after 7–10 weeks30, 31, 32 when hyperglycemia developed. In our study, lower serum insulin level of 16-week-ZDF rats was confirmed at 1.02 ng/ml, which is consistent with previous reported levels in 14- to 17-week-old ZDF.33, 34 Serum insulin level of 16-week-ZL rats (2.39 ng/ml) was significantly higher than that of ZDF, similar as reported previously in 12- to 16-week-ZL rats.35, 36, 37 Serum insulin level of 16-week-ZF rats (6.30 ng/ml) was significantly higher than both ZDF and ZL rats.38, 39, 40 The abnormally lower serum insulin level in ZDF rats is closely related to H2S metabolism. Both expression of CSE and H2S production rate in pancreatic islets were significantly higher in ZDF than in ZL or ZF rats (Figure 1b and c). Serum H2S level of ZDF was also significantly higher than that of ZF or ZL rats (Figure 5b). A greater pancreatic H2S production would underlie reduced insulin release from pancreatic β-cells of ZDF rats. Molecular basis for H2S-suppressed insulin release appears to be activation of KATP channels by high level of endogenous H2S in pancreatic β cells. This notion has been previously demonstrated by our group on INS-1E cells,11 and further confirmed in the present study on freshly isolated rat pancreatic β-cells. H2S at a physiologically relevant concentration increased the amplitude of whole-cell KATP channel currents in these β-cells. It should be pointed out that the demonstration of KATP activation by H2S in β-cells from Zucker rats does not exclude other potential pancreatic targets of H2S in insulin release process. Altered cellular redox status by H2S would be one example of these targets. Low levels of H2S (30–50 μM) decreased reactive oxygen species (ROS) in cultured vascular smooth muscle cells.41 Moreover, high level of NaHS (a donor of H2S) at 0.5 mM has been shown to deplete glutathione in isolated hepatocytes and increase hepatocyte formation of ROS, leading to cytotoxicity.42 Taking the same token, increased endogenous level of pancreatic H2S production in ZDF rats might increase ROS level and decrease β-cell mass. We have also previously demonstrated a proapoptotic effect of H2S on INS-1E cells. H2S increased ER stress of INS-1E cells and suppression of ER stress significantly decreased H2S-induced apoptosis.43
To further draw a correlation among pancreatic H2S metabolism, insulin release, and development of hyperglycemia, ZDF rats were treated with PPG. The inhibition of CSE activity by PPG in ZDF rats directly led to negligible pancreatic H2S production, further confirming the role of CSE as the dominant H2S-generating enzyme in pancreatic islet tissues. Residual H2S content in serum after PPG treatment indicates that H2S production remains functional in other tissues where CBS is the major H2S-producing enzyme. Diabetes is featured with failure to appropriately handle excess sugar presented after acute glucose uptake. Our present study revealed that PPG treatment improved glucose tolerance of ZDF rats (Figure 4a), which can be explained by improved insulin release in these rats (Figure 4b), because of reduced pancreatic H2S production. PPG treatment significantly lowered hyperglycemia and high HbA1c level of ZDF rats to near normal levels. These antidiabetic effects of PPG can be attributed to suppression of the inhibitory effect of H2S on pancreatic insulin release. To this end, decreased insulin release by high endogenous level of H2S as happened in untreated ZDF rats and increased insulin release by low endogenous level of H2S as happened in PPG-treated ZDF rats have been demonstrated. One limitation of this study, however, is that PPG treatment was conducted only at one concentration. Whether at this concentration PPG produces nonspecific effect on insulin release independent of the inhibition of CSE cannot be concluded yet.
A critical role of endogenous H2S in pancreatic islets in regulation of insulin release is unmasked in the present study. Abnormally elevated CSE gene expression and increased H2S production might constitute one of pathogenic mechanisms for diabetes. In this context, a hypothetic model of ‘sweetened rotten egg’ for physiological regulation of pancreatic function is proposed. This model integrates the role of pancreas in regulation of ‘sweet’ glucose metabolism and inhibitory role of the ‘rotten egg’ gas H2S in insulin release. As abnormal insulin release is ubiquitous for many disorders of insulin resistance syndrome, including obesity and hypertension, a critical evaluation of the role of pancreas as a ‘sweetened rotten egg’ will shed light on understanding of pathogenesis and management of these disorders.
References
Wang R . Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002;16:1792–1798.
Dello Russo C, Tringali G, Ragazzoni E, et al. Evidence that hydrogen sulphide can modulate hypothalamo–pituitary–adrenal axis function: in vitro and in vivo studies in the rat. J Neuroendocrinol 2002;12:225–233.
Zhao W, Zhang J, Lu Y, et al. H2S is an endogenous KATP channel opener in vascular smooth muscle cells. EMBO J 2001;20:6008–6016.
Zhao W, Wang R . H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol 2002;283:H474–H480.
Cheng Y, Ndisang JF, Tang G, et al. Sensitive relaxant response of rat mesenteric artery beds to hydrogen sulfide. Am J Physiol 2004;287:H2316–H2323.
Xiaohui L, Junbao D, Lin S, et al. Down-regulation of endogenous hydrogen sulfide pathway in pulmonary hypertension and pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats. Circ J 2005;69:1418–1424.
Wang R . We are what we smell—not only rotten eggs. Lab Invest 2006;86:324–325.
Yang G, Sun X, Wang R . Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of MAP kinases and caspase-3. FASEB J 2004;18:1782–1784.
Yang G, Cao K, Wang R . Cystathionine γ-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J Biol Chem 2004;279:49199–49205.
Deplancke B, Gaskins HR . Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J 2003;17:1310–1312.
Yang W, Yang G, Jia X, et al. Activation of KATP channels by H2S in insulin-secreting cells and the underlying mechanisms. J Physiol (Lond) 2005;569:519–531.
Yusuf M, Kwong Huat BT, Hsu A, et al. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulphide biosynthesis. Biochem Biophys Res Commun 2005;333:1146–1152.
Tamizhselvi R, Moore PK, Bhatia M . Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: in vitro studies using isolated mouse pancreatic acinar cells. J Cell Mol Med 2007;11:315–326.
Ali MY, Whiteman M, Low CM, et al. Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J Endocrinol 2007;195:105–112.
Kaneko Y, Kimura Y, Kimura H, et al. L-Cysteine inhibits insulin release from the pancreatic β-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes 2006;55:1391–1397.
Zhao W, Ndisang JF, Wang R . Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 2003;81:848–853.
Tang G, Wu L, Liang W, et al. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulphide in vascular smooth muscle cells. Mol Pharmacol 2005;68:1757–1764.
Khan SU, Morris GF, Hidiroglou M . Rapid estimation of sulfide in rumen and blood with a sulfide-specific ion electrode. Microchem J 1980;25:388–395.
Liang Y, Jetton TL, Lubkin M, et al. Bromocriptine/SKF38393 ameliorates islet dysfunction in the diabetic (db/db) mouse. Cell Mol Life Sci 1988;54:703–711.
Wicksteed B, Alarcon C, Briaud I, et al. Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet beta-cells but not regulated via a positive feedback of secreted insulin. J Biol Chem 2003;278:42080–42090.
Fakhrai-Rad H, Nikoshkov A, Kamel A, et al. Insulin-degrading enzyme identified as a candidate diabetes susceptibility gene in GK rats. Hum Mol Genet 2000;9:2149–2158.
Shu PY, Chang SF, Kuo YC, et al. Development of group- and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus. J Clin Microbiol 2003;41:2408–2416.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001;25:402–408.
Sun X, Cao K, Yang G, et al. Selective expression of Kir6.1 protein in different vascular and non-vascular tissues. Biochem Pharmacol 2004;67:147–156.
Joseph JW, Koshkin V, Zhang C-Y, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes 2002;51:3211–3219.
Wang R, Wu L . The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem 1997;272:8222–8226.
Ashcroft FM, Rorsman P . Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 1989;54:87–143.
Ashcroft SJH . The beta-cell KATP channel. J Membr Biol 2000;176:187–206.
Maechler P, Wollheim CB . Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 1999;402:685–689.
Clark JB, Palmer CJ, Shaw WN . The diabetic Zucker fatty rat. Proc Soc Exp Biol Med 1983;173:68–75.
Sparks JD, Phung TL, Bolognino M, et al. Lipoprotein alterations in 10- and 20-week-old Zucker diabetic fatty rats: hyperinsulinemic versus insulinopenic hyperglycemia. Metabolism 1988;47:1315–1324.
Johnson JH, Ogawa A, Chen L, et al. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science 1990;250:546–549.
Johns DG, Ao Z, Eybye M, et al. Rosiglitazone protects against ischemia/reperfusion-induced leukocyte adhesion in the zucker diabetic fatty rat. J Pharmacol Exp Ther 2005;315:1020–1027.
Gram DX, Hansen AJ, Deacon CF, et al. Sensory nerve desensitization by resiniferatoxin improves glucose tolerance and increases insulin secretion in Zucker diabetic fatty rats and is associated with reduced plasma activity of dipeptidyl peptidase IV. Eur J Pharmacol 2005;509:211–217.
Laight DW, Desai KM, Gopaul NK, et al. Pro-oxidant challenge in vivo provokes the onset of NIDDM in the insulin resistant obese Zucker rat. Br J Pharmacol 1999;128:269–271.
Hui H, Farilla L, Merkel P, et al. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. Eur J Endocrinol 2002;146:863–869.
Buhl ES, Jessen N, Pold R, et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes 2002;51:2199–2206.
Alemzadeh R, Holshouser S . Effect of diazoxide on brain capillary insulin receptor binding and food intake in hyperphagic obese Zucker rats. Endocrinology 1999;140:3197–3202.
Cettour-Rose P, Rohner-Jeanrenaud F . The leptin-like effects of 3-d peripheral administration of a melanocortin agonist are more marked in genetically obese Zucker (fa/fa) than in lean rats. Endocrinology 2002;143:2277–2283.
Mine T, Miura K, Kitahara Y, et al. Nateglinide suppresses postprandial hypertriglyceridemia in Zucker fatty rats and Goto-Kakizaki rats: comparison with voglibose and glibenclamide. Biol Pharm Bull 2002;25:1412–1416.
Yan SK, Chang T, Wang H, et al. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun 2006;351:485–491.
Truong DH, Eghbal MA, Hindmarsh W, et al. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 2006;38:733–744.
Yang G, Yang W, Wu L, et al. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem 2007;282:16567–16576.
Acknowledgements
This study has been supported by Natural Sciences and Engineering Research Council of Canada. L Wu is a New Investigator of Canadian Institutes of Health Research (CIHR). W Yang is supported by a post-doctoral fellowship from GREAT program of CIHR/Heart and Stroke Foundation of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, L., Yang, W., Jia, X. et al. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest 89, 59–67 (2009). https://doi.org/10.1038/labinvest.2008.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2008.109
Keywords
This article is cited by
-
The role of rhoA/rho-kinase and PKC in the inhibitory effect of L-cysteine/H2S pathway on the carbachol-mediated contraction of mouse bladder smooth muscle
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Targeting hepatic sulfane sulfur/hydrogen sulfide signaling pathway with α-lipoic acid to prevent diabetes-induced liver injury via upregulating hepatic CSE/3-MST expression
Diabetology & Metabolic Syndrome (2022)
-
A Facile Probe for Fluorescence Turn-on and Simultaneous Naked-Eyes Discrimination of H2S and biothiols (Cys and GSH) and Its Application
Journal of Fluorescence (2022)
-
Detection of hydrogen sulfide using BODIPY based colorimetric and fluorescent on-off chemosensor
Journal of Chemical Sciences (2020)
-
Gut microbial metabolites in obesity, NAFLD and T2DM
Nature Reviews Endocrinology (2019)