Abstract
Objective:
The objective of this study is to estimate the accuracy of early oral glucose tolerance testing (GTT), to predict impaired glucose tolerance.
Study Design:
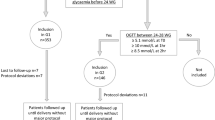
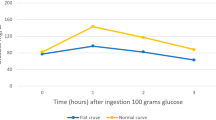
This was a prospective cohort study. Women received an early 75 g 2 h GTT between postpartum days 2–4 and again 6–12 weeks postpartum. The ability of the early GTT to accurately detect impaired glucose tolerance and diabetes was assessed by calculating sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPVs). The routine 6–12-week postpartum GTT was considered the gold standard.
Results:
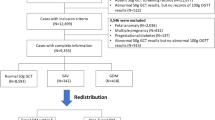
The early GTT was completed by 100% of subjects, whereas only 31 of 58 (53%) women returned to complete the 6–12-week postpartum GTT. The early GTT had modest sensitivity for impaired glucose tolerance (62.5%) and overt diabetes (50%). However, it had excellent specificity (100%), PPV (100%) and NPV (96.7%) for diabetes. The NPV for impaired glucose tolerance with the early GTT was 80%.
Conclusion:
Rates of 6–12 week postpartum GTT completion among patients with gestational diabetes is poor. Appropriate postpartum management may improve by using the early GTT as a screening test.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hogan P, Dall T, Nikolov P . American diabetes A. Economic costs of diabetes in the US in 2002. Diabetes care 2003; 26 (3): 917–932.
Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJG . Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metabol 2010; 95 (2): 670–677.
Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C . Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care 2011; 34 (7): 1582–1584.
Practice Bulletin No. 137. Gestational diabetes mellitus. Obstet Gynecol 2013; 122 (2 Pt 1): 406–416.
World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. WHO Document Production Services: Geneva, Switzerland, 2006.
National Institute for Health and Clinical Excellence (NICE) National Collaborating Centre for Women’s and Children’s Health. Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the postnatal period. National Institute for Health and Clinical Excellence (NICE) National Collaborating Centre for Women’s and Children’s Health. RCOG Press: London, 2008.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008; 31 (suppl. 1): S55–S60.
Committee on Obstetric Practice. ACOG committee opinion no. 435: postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus. Obstet Gynecol 2009; 113 (6): 1419–1421.
Genuth S, KG Alberti, Bennett P, Buse J, DeFronzo R, Kahn R et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26 (11): 3160–3167.
Metzger BE, Buchanan TA, Coustan DR, De Leiva A, Dunger DB, Hadden DR et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007; 30 (suppl. 2): S251–S260.
Korpi-Hyövälti E, Laaksonen DE, Schwab U, Heinonen S, Niskanen L . How can we increase postpartum glucose screening in women at high risk for gestational diabetes mellitus? Int J Endocrinol 2012; 2012: 519267.
Hunsberger ML, Donatelle RJ, Lindsay K, Rosenberg KD . Physician care patterns and adherence to postpartum glucose testing after gestational diabetes mellitus in Oregon. PLoS ONE 2012; 7 (10): e47052.
Shea AK, Shah BR, Clark HD, Malcolm J, Walker M, Karovitch A et al. The effectiveness of implementing a reminder system into routine clinical practice: does it increase postpartum screening in women with gestational diabetes? Chronic Dis Can 2011; 31 (2): 58–64.
Tovar A, Chasan-Taber L, Eggleston E, Oken E . Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis 2011; 8 (6): A124.
Newbern D, Freemark M . Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes 2011; 18 (6): 409–416.
Tuuli MG, Liu J, Stout MJ, Martin S, Cahill AG, Odibo AO et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med 2016; 374 (7): 647–655.
Werner EF, Has P, Tarabulsi G, Lee J, Satin A . Early postpartum glucose testing in women with gestational diabetes mellitus. Am J Perinatol 2016; 33 (10): 966–971.
Acknowledgements
EBC was supported by a NIH T32 training grant (5T32HD055172-05). Support for this research was provided in part by the Robert Wood Johnson Foundation. The views expressed here do not necessarily reflect the views of the Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Carter, E., Martin, S., Temming, L. et al. Early versus 6–12 week postpartum glucose tolerance testing for women with gestational diabetes. J Perinatol 38, 118–121 (2018). https://doi.org/10.1038/jp.2017.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2017.159