Abstract
Objective:
To evaluate the association between early pregnancy lipid profiles and pregnancy outcomes.
Study Design:
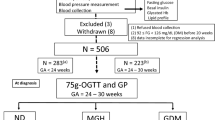
Retrospective 6 months analysis of 5218 singleton pregnant women. Each participant’s demographic and medical data were collected by questionnaires and medical records. Total cholesterol (TC), triglycerides (TG), high-density lipid cholesterol (HDL-C) and low-density lipid cholesterol (LDL-C) levels were divided into quartiles, and the women were categorized as having low (<25th percentile), referent (25 to <75th percentile) or high (>75th percentile) TC, TG, HDL-C and LDL-C values. Differences between groups were tested using t-test and Pearson's χ2-test. Binary logistic regression and multivariate analysis was conducted to evaluate the associations between lipid values and the risk of pregnancy outcomes.
Results:
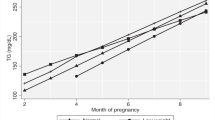
(1) Women who subsequently developed adverse pregnancy outcomes had higher levels of TC, TG, LDL-C and lower levels of HDL-C during early pregnancy (<14 gestational weeks). (2) A trend toward an increasing incidence of adverse pregnancy outcomes was noted with increasing levels of TC, TG and LDL-C, and decreasing level of HDL-C. (3) The more numbers of TC, TG and LDL-C above 75th percentile and HDL-C inferior to 25th percentile women had, the higher their risk of developing adverse pregnancy outcomes. (4) Low TG level was a protective factor for gestational diabetes mellitus (GDM) (<1.44 mmol l−1, odds ratio (OR)=0.706, 95% confidence interval (CI), 0.586 to 0.852) and large for gestational age infants (LGA) (<1.44 mmol l−1, OR=0.769, 95% CI, 0.631 to 0.936), and low LDL-C (<1.89 mmol l−1) level was protective factor for preterm birth. High TG (>1.40 mmol l−1, OR=1.327, 95% CI, 1.130 to 1.558), TC (>4.29 mmol l−1, OR=1.250, 95% CI, 1.062 to 1.471), and LDL-C (>2.62 mmol l−1, OR=1.25, 95% CI, 1.069 to 1.480) levels and a low HDL-C (<1.89 mmol l−1, OR=1.190, 95% CI, 1.007 to 1.405) level were associated with increased risk of GDM. A high TG (>1.40 mmol l−1, OR=1.550, 95% CI, 1.025 to 2.343) level was related to high risk of pre-eclampsia (PE), while a high LDL-C (>2.62 mmol l−1, OR=1.400, 95% CI, 1.100 to 1.781) level was risk factor for macrosomia. (5) After adjusting for confounders, early pregnancy TC was an independent risk factor for GDM (ajusted odds ratio [aOR]=1.184, 95% CI, 1.085 to 1.291), TG level was independently associated with the prevalence of GDM (aOR=1.253, 95% CI, 1.141 to 1.375) and PE (aOR=1.245, 95% CI, 1.023 to 1.516), and LDL-C level was significantly associated with risk of GDM (aOR=1.162, 95% CI, 1.052 to 1.283) and preterm birth (aOR=1.264, 95% CI, 1.065, 1.501).
Conclusions:
Early pregnancy high levels of TC, TG, LDL-C and low level of HDL-C may be predictive biomarkers for adverse pregnancy outcomes, while early pregnancy low TC, TG, LDL-C levels and high HDL-C levels could have some protective roles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Haugen AC, Schug TT, Collman G, Heindel JJ . Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis 2015; 6 (2): 55–64.
Fleming TP, Velazquez MA, Eckert JJ . Embryos, DOHaD and David Barker. J Dev Orig Health Dis 2015; 8: 1–7.
Tangvarasittichai S . Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015; 6 (3): 456–480.
Silva IT, Almeida-Pititto Bd, Ferreira SR . Reassessing lipid metabolism and its potentialities in the prediction of cardiovascular risk. Arch Endocrinol Metab 2015; 59 (2): 171–180.
Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF . Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG 2015; 122 (5): 643–651.
Wang D, Xu S, Chen H, Zhong L, Wang Z . The associations between triglyceride to high-density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large-for-gestational-age infant. Clin Endocrinol (Oxf) 2015; 83 (4): 490–497.
Spracklen CN, Smith CJ, Saftlas AF, Robinson JG, Ryckman KK . Maternal hyperlipidemia and the risk of preeclampsia: a meta-analysis. Am J Epidemiol 2014; 180 (4): 346–358.
Laughon SK, McLain AC, Sundaram R, Catov JM, Buck Louis GM . Maternal lipid change in relation to length of gestation: a prospective cohort study with preconception enrollment of women. Gynecol Obstet Invest 2014; 77 (1): 6–13.
Mossayebi E, Arab Z, Rahmaniyan M, Almassinokiani F, Kabir A . Prediction of neonates' macrosomia with maternal lipid profile of healthy mothers. Pediatr Neonatol 2014; 55 (1): 28–34.
Kramer MS, Kahn SR, Dahhou M, Otvos J, Genest J, Platt RW et al. Maternal lipids and small for gestational age birth at term. J Pediatr 2013; 163 (4): 983–988.
Tal R, Taylor HS, Burney RO, Mooney SB, Giudice LC .Endocrinology of Pregnancy.In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, et al. (eds). Source Endotext [Internet]. South Dartmouth, MA, USA: MDText.com, Inc.; 2000-2015.
Agarwal MM, Boulvain M, Coetzee E, Colagiuri S, Falavigna M, Hod M et al. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 2014; 103 (3): 341–363.
Pauli JM, Repke JT . Preeclampsia: Short-term and Long-term Implications. Obstet Gynecol Clin North Am 2015; 42 (2): 299–313.
Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384 (9946): 857–868.
Enquobahrie DA, Williams MA, Qiu C, Luthy DA . Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract 2005; 70 (2): 134–142.
Li G, Kong L, Zhang L, Fan L, Su Y, Rose JC et al. Early pregnancy maternal lipd profiles and the risk of gestational diabetes mellitus stratified for body mass index. Reprod Sci 2015; 22 (6): 712–717.
Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA . Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens 2004; 17 (7): 574–581.
Baumfeld Y, Novack L, Wiznitzer A, Sheiner E, Henkin Y, Sherf M et al. Pre-conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS ONE 2015; 10 (10): e0139164.
Vrijkotte TG, Algera SJ, Brouwer IA, van Eijsden M, Twickler MB . Maternal triglyceride levels during early pregnancy are associated with birth weight and postnatal growth. J Pediatr 2011; 159 (5): 736–742.
Vrijkotte TG, Krukziener N, Hutten BA, Vollebregt KC, van Eijsden M, Twickler MB . Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab 2012; 97 (11): 3917–3925.
Catov JM, Bodnar LM, Kip KE, Hubel C, Ness RB, Harger G et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol 2007; 197 6: 610.e1–7.
Khoury J, Henriksen T, Christophersen B, Tonstad S . Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. Am J Obstet Gynecol 2005; 193 (4): 1292–1301.
Abderrahmani A, Niederhauser G, Favre D, Abdelli S, Ferdaoussi M, Yang JY et al. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia 2007; 50 (6): 1304–1314.
Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G . Triglyceride metabolism in pregnancy. Adv Clin Chem 2011; 55: 133–153.
Guerby P, Vidal F, Garoby-Salom S, Vayssiere C, Salvayre R, Parant O et al. [Oxidative stress and preeclampsia: A review]. Gynecol Obstet Fertil 2015 pii S1297-9589 (15): 00270–00272.
Shevchuk SV, Seheda IuS, Kuvikova IP . Dyslipidemia in patients with antiphospholipid syndrome and its association with endothelial dysfunction and atherosclerotic changes in the carotid arteries. Lik Sprava 2013; 2: 38–47.
Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB . Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 2014; 15 (9): 16153–16185.
Freinkel N . Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980; 29 (12): 1023–1035.
Aucott L, Gray D, Rothnie H, Thapa M, Waweru C . Effects of lifestyle interventions and long-term weight loss on lipid outcomes - a systematic review. Obes Rev 2011; 12 (5): e412–e425.
Pu J, Romanelli R, Zhao B, Azar KM, Hastings KG, Nimbal V et al. Dyslipidemia in special ethnic populations. Endocrinol Metab Clin North Am 2016; 45 (1): 205–216.
Acknowledgements
The study was supported by ‘China GDM centers-Establishment and Training Dissemination’ from the World Diabetes Foundation (Grant number WDF10-517) and ‘Diabetes Management beyond Pregnancy’ via the World Diabetes Foundation (Grant number WDF14-908) and ‘Sample library construction and plasma biomarker investigation on recurrent spontaneous abortion and preeclampsia’ from the Major Program of the National Natural Science Foundation of China (81490745) and 973 national science and technology plan project (2015CB94330). We thank all the obstetricians and students in our research group for data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, C., Zhu, W., Wei, Y. et al. The associations between early pregnancy lipid profiles and pregnancy outcomes. J Perinatol 37, 127–133 (2017). https://doi.org/10.1038/jp.2016.191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.191
This article is cited by
-
The association between dyslipidaemia in the first trimester and adverse pregnancy outcomes in pregnant women with subclinical hypothyroidism: a cohort study
Lipids in Health and Disease (2024)
-
Consecutive reference intervals for biochemical indices related to serum lipid levels and renal function during normal pregnancy
BMC Pregnancy and Childbirth (2022)
-
Early postpartum abnormal glucose metabolism subtype differs according to mid-trimester lipid profile in women with gestational diabetes mellitus
Lipids in Health and Disease (2021)
-
Second-trimester and third-trimester maternal lipid profiles significantly correlated to LGA and macrosomia
Archives of Gynecology and Obstetrics (2021)
-
The relationship between total cholesterol and postpartum impaired glucose tolerance in women with gestational diabetes mellitus
Lipids in Health and Disease (2020)