Abstract
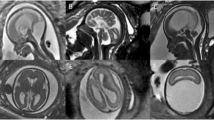
Fetal and neonatal brain tumors are rare. Prenatal ultrasound aids early tumor detection. Nonetheless, we encountered a preterm neonate born at 32 weeks gestation with a massive supratentorial glioma, which was undetected on ultrasound at 19-6/7 weeks gestation. The patient presented at birth with unanticipated massive macrocephaly. Resuscitation and stabilization were difficult, but the medical team felt that futility of care was not established and opted to transfer the baby to an academic center for further imaging and specialist consultations. Diagnosis of an extensive, inoperable tumor was confirmed and support withdrawn. Postmortem histologic examination and immunohistochemical stains identified the majority of tumor cells as glial in origin. This case report illustrates well how a severe and potentially fatal anomaly, which remained undetected prenatally, presented the medical team and family with multiple medical, ethical and emotional challenges at birth; decisions regarding futility of care in the neonatal transport setting are difficult.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Isaacs H Jr . I. Perinatal brain tumors: a review of 250 cases. Pediatr Neurol 2002; 27: 249–261.
Isaacs H Jr . II. Perinatal brain tumors: a review of 250 cases. Pediatr Neurol 2002; 27: 333–342.
Kamil D, Tepelmann J, Berg C, Heep A, Axt-Fliedner R, Gembruch U et al. Spectrum and outcome of prenatally diagnosed fetal tumors. Ultrasound Obstet Gynecol 2008; 31: 296–302.
Nejat F, Kazmi SS, Ardakani SB . Congenital brain tumors in a series of seven patients. Pediatr Neurosurg 2008; 44: 1–8.
Hwang SW, Su JM, Jea A . Diagnosis and management of brain and spinal cord tumors in the neonate. Semin Fetal Neonatal Med 2012; 17: 202.
Cavalheiro S, Moron AF, Hisaba W, Dastoli P, Silva NS . Fetal brain tumors. Child's Nerv Syst 2003; 19: 529–536.
Manoranjan B, Provias JP . Congenital brain tumors: diagnostic pitfalls and therapeutic interventions. J Child Neurol 2011; 26: 599–614.
Shamji MF, Vassilyadi M, Lam CH, Montes JL, Farmer JP . Congenital tumors of the central nervous system: the MCH experience. Pediatr Neurosurg 2009; 45: 368–374.
Schlembach D, Bornemann A, Rupprecht T, Beinder E . Fetal intracranial tumors detected by ultrasound: a report of two cases and review of the literature. Ultrasound Obstet Gynecol 1999; 14: 407–418.
Alvarez M, Chitkara U, Lynch L, Mehalek KE, Heller D, Berkowitz RL . Prenatal diagnosis of fetal brain tumors. Fetal Ther 1987; 2: 203–208.
Saada J, Enza-Razavi F, Delahaye S, Martinovic J, Macaleese J, Benachi A . Early second-trimester diagnosis of intracranial teratoma. Ultrasound Obstet Gynecol 2009; 33: 109–111.
Wilkinson D, de Crespigny L, Xafis V . Ethical language and decision-making for prenatally diagnosed lethal malformations. Semin Fetal Neonatal Med 2014; 19: 306–311.
Wilkinson DJ . Futile treatment: the ethicist's perspective. Med J Aust 2013; 198: 223–224.
Feltman D, Stokes T, Kett J, Lantos JD . Is treatment futile for an extremely premature infant with giant omphalocele? Pediatrics 2014; 133: 123–128.
Fine RL, Whitfield JM, Carr BL, Mayo TW . Medical futility in the neonatal intensive care unit: hope for a resolution. Pediatrics 2005; 116: 1219–1222.
Dulkerian SJ, Douglas WP, Taylor RM . Redirecting treatment during neonatal transport. J Perinat Neonatal Nurs 2011; 25: 111–114.
Meadow W, Cohen-Cutler S, Spelke B, Kim A, Plesac M, Weis K et al. The prediction and cost of futility in the NICU. Acta Paediatr 2012; 101: 397–402.
Acknowledgements
We acknowledge the staff of the referring hospital, transport team and receiving hospital (who are not further named to deidentify the patient). We thank Dr Judith Rossiter for review of the deidentified prenatal US findings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have not received any support for the work in the form of grants and/or equipment or drugs. The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Olischar, M., Stavroudis, T., Karp, J. et al. Medical and ethical challenges in the case of a prenatally undiagnosed massive congenital brain tumor. J Perinatol 35, 773–775 (2015). https://doi.org/10.1038/jp.2015.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.80