Abstract
Objective:
To evaluate efficacy of predischarge transcutaneous bilirubin (TcB) measurement and clinical risk assessment in predicting hyperbilirubinemia needing treatment.
Study Design:
A diagnostic test was performed in a prospective cohort study conducted at a teaching hospital in North India. Subjects included healthy neonates with a gestation period of ⩾35 weeks or birth weight ⩾2000 g. Maternal, neonatal and delivery risk factors for hyperbilirubinemia were prospectively collected. TcB was measured in all enrolled neonates at 24±6, 72 to 96 and 96 to 144 h of postnatal age and when indicated clinically. Neonates were followed up during hospital stay and after discharge till completion of the 7th postnatal day. The key outcome was significant hyperbilirubinemia defined as need of phototherapy on the basis of modified American Academy of Pediatrics guidelines. In neonates born at ⩾38 weeks of gestation and in neonates born at ⩽37 completed weeks of gestation, middle line and lower line of phototherapy thresholds were used to initiate phototherapy, respectively. Variables observed to be significantly associated with significant hyperbilirubinemia on multivariate analysis were used for construction of a clinical risk assessment tool. Predictive ability of the risk assessment tool was assessed by calculating sensitivity, specificity, positive predictive value and negative predictive value, by plotting receiver-operating characteristics curve and calculating c-statistic.
Result:
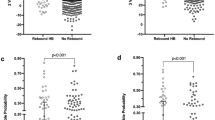
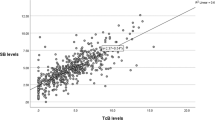
A total of 997 neonates (birth weight: 2627±536 g, gestation: 37.8±1.5 weeks) were enrolled in the study, of which 931 completed follow-up. Among enrolled neonates, 344 (34.5%) were low birth weight. Overall, a total of 199 (20%) neonates developed significant hyperbilirubinemia. On stepwise logistic regression analysis, predischarge TcB percentile and gestation were significantly found to be associated with significant hyperbilirubinemia. A risk assessment graph was constructed to predict subsequent development of significant hyperbilirubinemia. Area under curve for this risk assessment strategy was 0.75.
Conclusion:
A risk assessment graphical tool consisting of TcB and gestation accurately predicted subsequent need of phototherapy. Further studies are needed to validate performance of this risk assessment tool.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Report 2002–2003: National Neonatal Perinatal Database Network. National Neonatology Forum of India, New Delhi, India, 2004.
Watchko JF . Identification of neonates at risk for hazardous hyperbilirubinemia: emerging clinical insights. Pediatr Clin North Am 2009; 56 (3): 671–687.
Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004; 114 (1): 297–316.
Lodha R, Deorari AK, Jatana V, Paul VK . Non-invasive estimation of total serum bilirubin by multi-wavelength spectral reflectance in neonates. Indian Pediatr 2000; 37 (7): 771–775.
Agarwal R, Kaushal M, Aggarwal R, Paul VK, Deorari AK . Early neonatal hyperbilirubinemia using first day serum bilirubin level. Indian Pediatr 2002; 39 (8): 724–730.
Stevenson DK, Fanaroff AA, Maisels MJ, Young BW, Wong RJ, Vreman HJ . et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics 2001; 108 (1): 31–39.
Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH . Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000; 106 (2): E17.
Bhutani VK, Johnson L, Sivieri EM . Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999; 103 (1): 6–14.
Ip S, Chung M, Kulig J, O’Brien R, Sege R, Glicken S et al. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics 2004; 114 (1): e130–e153.
Trikalinos TA, Chung M, Lau J, Ip S . Systematic review of screening for bilirubin encephalopathy in neonates. Pediatrics 2009; 124 (4): 1162–1171.
Mishra S, Chawla D, Agarwal R, Deorari AK, Paul VK, Bhutani VK . Transcutaneous bilirubinometry reduces the need for blood sampling in neonates with visible jaundice. Acta Paediatr 2009; 98 (12): 1916–1919.
Carley S, Dosman S, Jones SR, Harrison M . Simple nomograms to calculate sample size in diagnostic studies. Emerg Med J 2005; 22 (3): 180–181.
Varvarigou A, Fouzas S, Skylogianni E, Mantagou L, Bougioukou D, Mantagos S . Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics 2009; 124 (4): 1052–1059.
Maisels MJ, Kring E . Transcutaneous bilirubin levels in the first 96 h in a normal newborn population of > or = 35 weeks’ gestation. Pediatrics 2006; 117 (4): 1169–1173.
Bhat YR, Rao A . Transcutaneous bilirubin in predicting hyperbilirubinemia in term neonates. Indian J Pediatr 2008; 75 (2): 119–123.
Keren R, Bhutani VK, Luan X, Nihtianova S, Cnaan A, Schwartz JS . Identifying newborns at risk of significant hyperbilirubinaemia: a comparison of two recommended approaches. Arch Dis Child 2005; 90 (4): 415–421.
Chou SC, Palmer RH, Ezhuthachan S, Newman C, Pradell-Boyd B, Maisels MJ et al. Management of hyperbilirubinemia in newborns: measuring performance by using a benchmarking model. Pediatrics 2003; 112 (6 Part 1): 1264–1273.
Newman TB, Xiong B, Gonzales VM, Escobar GJ . Prediction and prevention of extreme neonatal hyperbilirubinemia in a mature health maintenance organization. Arch Pediatr Adolesc Med 2000; 154 (11): 1140–1147.
Newman TB, Liljestrand P, Escobar GJ . Combining clinical risk factors with serum bilirubin levels to predict hyperbilirubinemia in newborns. Arch Pediatr Adolesc Med 2005; 159 (2): 113–119.
Lo SF, Doumas BT . The status of bilirubin measurements in U.S. laboratories: why is accuracy elusive? Semin Perinatol 2011; 35 (3): 141–147.
Vreman HJ, Verter J, Oh W, Fanaroff AA, Wright LL, Lemons JA et al. Interlaboratory variability of bilirubin measurements. Clin Chem 1996; 42 (6 Part 1): 869–873.
Mishra S, Chawla D, Agarwal R, Deorari AK, Paul VK . Transcutaneous bilirubin levels in healthy term and late preterm Indian neonates. Indian J Pediatr 2010; 77 (1): 45–50.
Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF . Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics 2009; 124 (4): 1193–1198.
Murki S, Kumar P, Majumdar S, Marwaha N, Narang A . Risk factors for kernicterus in term babies with non-hemolytic jaundice. Indian Pediatr 2001; 38 (7): 757–762.
Agrawal VK, Shukla R, Misra PK, Kapoor RK, Malik GK . Brainstem auditory evoked response in newborns with hyperbilirubinemia. Indian Pediatr 1998; 35 (6): 513–518.
Kumar P, Jain N, Thakre R, Murki S, Venkataseshan S (eds). Evidence Based Clinical Practice Guidelines. National Neonatology Forum of India, New Delhi, India, 2010.
Acknowledgements
We thank Mr Ravinder Kumar, Medical Social Worker for helping in ensuring follow-up of the enrolled neonates.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaur, S., Chawla, D., Pathak, U. et al. Predischarge non-invasive risk assessment for prediction of significant hyperbilirubinemia in term and late preterm neonates. J Perinatol 32, 716–721 (2012). https://doi.org/10.1038/jp.2011.170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2011.170
Keywords
This article is cited by
-
Bilirubin nomograms for identification of neonatal hyperbilirubinemia in healthy term and late-preterm infants: a systematic review and meta-analysis
World Journal of Pediatrics (2014)
-
Routine blood typing and DAT in infants of group O mothers
Journal of Perinatology (2013)