Abstract
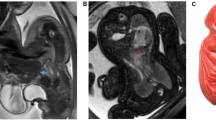
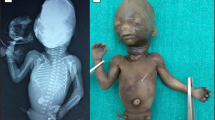
Monozygotic twins with discordant karyotypes are rare. We report a case of monozygotic twins discordant for trisomy 13 by amniocyte karyotypes. Ultrasound revealed multiple congenital anomalies in Twin A (47,XY,+13), none in Twin B (46,XY), and monochorionic-diamniotic placentation. Zygosity testing performed both prenatally and after birth supported monozygosity. Twin A died in the neontal period. Twin B survived and had normal physical examination, but peripheral blood karyotype revealed 20% mosaicism for trisomy 13. Monochorionic-diamniontic placentation with vascular anastomoses was confirmed by pathological examination. In this paper, we discuss the various mechanisms by which monozygotic twins may have discordant karyotypes. The surviving twin, structurally and developmentally normal at 6 months of age, will be monitored for potential complications of uniparental disomy of chromosome 13 and trisomy 13 mosaicism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Redline RW . Nonidentical twins with a single placenta—disproving dogma in prenatal pathology. N Engl J Med 2003; 349 (2): 111–114.
Cheng PJ, Shaw SW, Shih JC, Soong YK . Monozygotic twins discordant for monosomy 21 detected by first-trimester nuchal translucency screening. Obstet Gynecol 2006; 107 (2 Part 2): 538–541.
Dahoun S, Gagos S, Gagnebin M, Gehrig C, Burgi C, Simon F et al. Monozygotic twins discordant for trisomy 21 and maternal 21q inheritance: a complex series of events. Am J Med Genet A 2008; 146A (16): 2086–2093.
Nieuwint A, Van Zalen-Sprock R, Hummel P, Pals G, Van Vugt J, Van Der Harten H et al. ‘Identical’ twins with discordant karyotypes. Prenat Diagn 1999; 19 (1): 72–76.
Rogers JG, Voullaire L, Gold H . Monozygotic twins discordant for trisomy 21. Am J Med Genet 1982; 11 (2): 143–146.
O’Donnell CP, Pertile MD, Sheffield LJ, Sampson A . Monozygotic twins with discordant karyotypes: a case report. J Pediatr 2004; 145 (3): 406–408.
Taylor DM, Thum MY, Abdalla H . Dichorionic triamniotic triplet pregnancy with monozygotic twins discordant for trisomy 13 after preimplantation genetic screening: case report. Fertil Steril 2008; 90 (5): 2017–e2015-2019.
Sepulveda W, Wong AE, Ocaranza M . Heterokaryotypic pregnancy: monozygotic monochorionic twins discordant for trisomy 13. Fetal Diagn Ther 2010; 28 (2): 109–113.
Machin G . Non-identical monozygotic twins, intermediate twin types, zygosity testing, and the non-random nature of monozygotic twinning: a review. Am J Med Genet C Semin Med Genet 2009; 151C (2): 110–127.
Berend SA, Feldman GL, McCaskill C, Czarnecki P, Van Dyke DL, Shaffer LG . Investigation of two cases of paternal disomy 13 suggests timing of isochromosome formation and mechanisms leading to uniparental disomy. Am J Med Genet 1999; 82 (3): 275–281.
Soler A, Margarit E, Queralt R, Carrio A, Costa D, Gomez D et al. Paternal isodisomy 13 in a normal newborn infant after trisomy rescue evidenced by prenatal diagnosis. Am J Med Genet 2000; 90 (4): 291–293.
Jarvela I, Savukoski M, Ammala P, von Koskull H . Prenatally detected paternal uniparental chromosome 13 isodisomy. Prenat Diagn 1998; 18 (11): 1169–1173.
Slater H, Shaw JH, Dawson G, Bankier A, Forrest SM . Maternal uniparental disomy of chromosome 13 in a phenotypically normal child. J Med Genet 1994; 31 (8): 644–646.
Walker SP, Meagher S, White SM . Confined blood chimerism in monochorionic dizygous (MCDZ) twins. Prenat Diagn 2007; 27 (4): 369–372.
Griffith CB, Vance GH, Weaver DD . Phenotypic variability in trisomy 13 mosaicism: two new patients and literature review. Am J Med Genet A 2009; 149A (6): 1346–1358.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ramsey, K., Slavin, T., Graham, G. et al. Monozygotic twins discordant for trisomy 13. J Perinatol 32, 306–308 (2012). https://doi.org/10.1038/jp.2011.123
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2011.123