Abstract
Objective:
To evaluate the efficacy of probiotics in the prevention of gastrointestinal colonization by Candida species, of late-onset sepsis and neurological outcome in preterm newborns.
Study Design:
A prospective study was conducted in 249 preterms who were subdivided into three groups: one group (n=83) was supplemented with Lactobacillus (L.) reuteri, one group with L. rhamnosus (n=83) and the other with no supplementation (n=83). The fungal colonization in the gastrointestinal tract, the late onset of sepsis and clinical parameters were recorded. A neurological structured assessment was further performed at 1 year of age.
Result:
Candida stool colonization was significantly higher (P<0.01) in the control group than in the groups treated with probiotics. The L. reuteri group presented a significantly higher reduction in gastrointestinal symptoms than did the L. rhamnosus and control groups. Infants treated with probiotics showed a statistically significant lower incidence of abnormal neurological outcome than did the control group.
Conclusion:
The use of both probiotics seems to be effective in the prevention of gastrointestinal colonization by Candida, in the protection from late-onset sepis and in reducing abnormal neurological outcomes in preterms.
Similar content being viewed by others
Introduction
The incidence of Candidemia in the neonatal intensive care unit (NICU) is steadily increasing, with an estimated incidence of 1.6 to 9% in very-low-birth-weight infants and of 15% in extremely low-birth-weight infants.1, 2, 3, 4 Low birth weight is an important risk factor in neonatal mortality and in various morbidities, because of the immaturity of both the immune system and the barrier functions of the gastrointestinal tract, as well as the invasive diagnostic and therapeutic procedures.1 In a report of the National Nosocomial Infections Surveillance System, Candida species were ranked second in frequency among blood culture isolates obtained from neonates in high-risk nurseries.2, 3, 4 Colonization by Candida species is also frequently associated with an increased risk for invasive fungal infections (IFI),5, 6 and it can always be found preliminarily to the development of IFI thanks to a careful investigation. The gastrointestinal tract seems to be the site most frequently implicated in systemic dissemination, as the incidence of rectal fungal colonization from several single-site studies of very-low-birth-weight infants is between 19.2 and 45% and it is higher (63%) when stool is cultured directly.7
Premature newborns in the intensive care setting harbor a bacterial flora that is greatly modified, composed of <20 species, with predominant aerobes such as Staphylococci (coagulase negative and Staphylococcus aureus), enterobacteria (Klebsiella) and enterococci, whereas the predominant anaerobes include Clostridia.8
A normal bacterial flora inhibits Candida growth by competing for both adhesion sites and nutrients.9 Antibiotic therapy and infective events induce rapid changes in the intestinal microflora, whereas hygienic conditions and nutrition induce slow changes. The use of H2 blockers is considered a further risk factor for Candida infection.10 The direct introduction of particular microbiological strains into the diet, in particular those known as ‘probiotics,’ can positively influence the intestinal microbial population.11 The bacteria most frequently used as probiotics are the bifidobacteria and certain strains of lactobacilli, including Lactobacillus (L.) reuteri and L. rhamnosus. Several trials have reported the beneficial effects of the use of these probiotics in reducing undesirable outcomes such as diarrhea, colon distension and abdominal cramps, but few clinical trials exist for preterm infants. Early comparative studies have reported data on the safety and potential colonization of probiotics in infants and on the impact of probiotic supplementation on their enteric microflora.12, 13, 14, 15, 16, 17
Therefore, the purposes of this were as follows: (1) to evaluate the hypothesis that supplementation with probiotics may reduce the colonization and expansion of fungal colonies in the gastrointestinal tract, and reduce the risk of bacterial and/or fungal nosocomial infections in the NICU; (2) to determine a possible difference according to birth weight; as newborns with candidiasis have reported a worse neuronal development than the control group:18 a further objective of this study was (3) to examine the effect of probiotic supplementation on clinical neurological outcomes in NICU newborns.
Methods
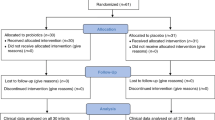
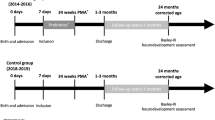
From January 2004 to December 2007, a prospective randomized trial was conducted in 249 preterms with a birth weight <2500 g and a gestational age <37 weeks consecutively admitted at the NICU of the Policlinico University of Catania (Italy), which is a level III neonatal center. All the infants were outborn. Inclusion criteria were admission to the NICU, a stable oral feeding within 72 h of birth and an informed parental consent; exclusion criteria were the presence of major congenital malformation or antenatal and perinatal risk factors for sepsis.
The newborns were randomized into three groups by a random number table: group I (n=83; 12 with a birth weight <1500 g, 71 ⩾1500 g) received supplementation with L. reuteri American Type Culture Collection (ATCC) 55730 5 drops daily; group II (n=83; 28 <1500 g, 55 ⩾1500 g) received supplementation with L. rhamnosus ATCC 53103 1 capsule daily; and group III included newborns with no probiotics (control; n=83; 16 <1500 g, 67 ⩾1500 g). Patients received supplementation from the first 72 h after hospitalization for 6 weeks or until they were discharged from the NICU.
Clinical procedures
The nutritional policy was conducted as an enteral feeding with breast milk or formula milk (1 ml every 3 h) through an orogastric tube within few hours after delivery. After the first day of life, parenteral nutrition was provided through a Premicath catheter; glucose, amino acids and lipids were administered from the second day of life. Nutrition administered through oral access with intermittent meals or continuous pump feeding was progressively increased, if tolerated, and parenteral nutrition was progressively decreased and stopped, following the same protocols during the study period, as reported by the international guidelines.19, 20
Infants were weighed daily, and the day on which parenteral nutritional was stopped was also recorded. All infants were carefully examined by the nurses at least twice daily for gastrointestinal symptoms,21 such as regurgitation (defined as the passage of refluxed gastric contents into the oral pharynx), vomiting (defined as the expulsion of the refluxed gastric contents from the mouth, that is, feeding intolerance), abdominal distension and characteristics of the feces. A hydrolyzed formula was used when newborns presented any gastrointestinal symptoms to improve the feeding tolerance. Length of hospitalization was also recorded.
Clinical signs of infection were monitored, including fever, desaturation crisis, apnea, bradycardia, pallor or grayish coloring, necessity of O2 supplementation and/or reintubation. The following laboratory parameters were also monitored cultures of organic liquids, C-reactive protein and blood count. Investigations to detect any mycotic involvement of the organs included ultrasounds (USs) (renal, cardiac, abdominal, transfontanellar), examination of the fundus oculi and chest X-rays.
Microbiology
Stool samples and gastric aspirate specimens were collected in sterile containers. Oropharyngeal specimens were collected on swabs and immediately transported to the laboratory situated in the same structure for processing.
Gastric aspirations and pharyngeal swabs were cultured for Candida detection at birth and after 7, 14, 21 and 28 days; quantitative fungal stool cultures were also examined at the same time.22 Infants were considered at high level of colonization if they presented >104 CFU (colony-forming unit) per gram of feces.7
Blood cultures and Platelia Candida test were conducted for the diagnosis of invasive candidiasis and to evaluate antifungal chemotherapy efficacy.23
Proven fungal or bacterial infection was defined as a positive culture: (1) from blood (drawn from peripheral sites); (2) from urine (collected by suprapubic sterile puncture or sterile bladder catheterization, with a growth of 10 000 fungal organisms per ml); (3) from cerebrospinal fluid; or (4) from intravascular catheter tip (only considered proof of microbiologically documented fungal infection in patients with previous peripheral colonization by the same species). During the study period, infection-control policies did not significantly differ in our Unit following the same criteria expressed by previously published protocols.24
Treatment
L. reuteri (ATCC 55730) was administered at the dose of 5 drops per day in an oil formulation, which delivered 1 × 108 CFU, through an orogastric tube or into the mouth directly.17, 25
L. rhamnosus (ATCC 53103) was administered at the dose of 1 capsule per day, equivalent to 6 × 109 CFU. The capsules were opened and contents dispersed in water before administering. Probiotics treatment continued until infants left the NICU.26
Antimycotic treatment consisted of liposomal Amphotericin B at the initial dose of 1 mg kg−1 per day, with a gradual increase up to a maximum of 6 mg kg−1 per day, by continuous infusions over 30 min.27 Treatment was stopped 7 days after a negative culture for Candida and three consecutive negative C-reactive protein and Candida antigen tests.
Antibiotic treatment was carried out after antibiotic assay with the following drugs: Teicoplanin, Vancocin, Ampicillin sulbactam, Amikacin, Imipenem, Meropenem. Treatment was stopped 7 to 12 days after negative assay of C-reactive protein and the absence of clinical signs of infection.28
Neurological assessment
The HINE (Hammersmith Infant Neurological examination) was conducted to assess the neurological status of all newborns at a corrected age of 12 months.29 This is a structured neurological examination, including the assessment of cranial nerve, posture, movements, tone and reflexes. An optimality score is obtained by calculating the distribution of the score frequency in the normal population, defining as optimal all the scores found in at least 90% of the cohort. At 12 months, the scores equal to or above 73 were regarded as optimal, and those below 73 as suboptimal.29
Cranial US was performed for each preterm infant in the first week of life and another between 15 and 21 days after birth and at least one at term age. An US scan was considered as abnormal if one of the following abnormalities was detected: echodensities persisting for more than 14 days without cyst formation, cystic periventricular leukomalacia, periventricular parenchymal hemorrhagic involvement and isolated ventricular dilatation.30
Ethics
Ethical approval was obtained from the ethics committee of our institution.
Statistical methods
The primary end point was to evaluate the incidence of enteric fungal colonization. The χ2 test was used to analyze the data along with Fisher's exact test when applicable. The anthropometric variables (weight at birth and gestational age) were reported as means±s.d. The intergroup comparisons (L. reuteri, L. rhamnosus, control) for all variables were performed by a nonparametric test (Kruskall–Wallis with a post hoc test of Dunn). The same analysis was carried out for the total population and for the newborns divided according to the birth weight, both <1500 and ⩾1500 g. The level of significance was set at P<0.05.
Results
The demographic characteristics of preterm infants are reported in Table 1, with a similar distribution in the three groups for all parameters. No significant differences at baseline between the three groups were seen for the presence of major risk factor (maternal antenatal steroid). In our study, we did not use postnatal steroid.
The group supplemented with L. reuteri presented a significant reduction in gastrointestinal symptoms (Table 2) than the L. rhamnosus and control group. Furthermore, the control group fed a higher percentage of hydrolyzed formula milk (Table 2) than both the probiotic groups.
The duration of parenteral nutrition (Table 2, Figure 1) was statistically different between L. reuteri and both L. rhamnosus and control group (P<0.0001), whereas there was not any statistically difference between the L. rhamnosus and the control groups. Similarly, the energy quotient (kcal kg−1) was quickly achieved in the L. reuteri group compared with the control and the L. rhamnosus group (P<0.0001) (Table 2, Figure 1)
Candida stool colonization was significantly higher (P<0.01) in the control group than in both probiotic groups (Table 2). The number of invasive Candida infections between the three groups was not statistically different. All the infants with IFI had a gestational age <32 weeks and a birth weight <1500 g. The site of isolation of all the Candida species was peripheral blood (Table 3). No statistical differences between the three groups were observed in the duration of antimycotic treatment. In infants affected by Candida infections, the antimycotic or antibiotic treatment associated with probiotic supplementation resulted in a fast clinical improvement, with fewer days of treatment in the two probiotic groups than in the control group (Table 2, Figure 2). The same was observed for bacterial infections (Table 2).
The use of L. reuteri led to a significant reduction in the number of days of hospitalization than the L. rhamnosus and the control groups (P<0.0001) (Table 2) (Figure 1).
In Table 2, the data of the population divided according to the birth weight are reported; the differences between the three groups observed in the total population were confirmed only for the newborns with a birth weight ⩾1500 g. For those with a birth weight <1500 g, the differences were significant between the L. reuteri and L. rhamnosus groups for the duration of parenteral nutrition, the days to achieve the energy quotient, the days of antibiotic treatment and the duration of hospitalization, with better results for the former. Furthermore, newborns treated with L. reuteri showed lesser number of days of antibiotic treatment and lesser duration of hospitalization than the control group.
Neurological assessment
In all, 202 out of 249 newborns showed a normal neurological assessment within the normal range of the optimal scores at the HINE; 47 presented suboptimal scores; no statistical differences were observed in the incidence of suboptimal scores in the groups of infants treated with probiotics (n=10 with L. reuteri, n=13 with L. rhamnosus; P>0.05). A statistically significant higher incidence of suboptimal scores (P<0.05) was observed in the control group (n=24) than in both the probiotic groups. A total of 40 infants reported an abnormal cranial US with no statistical differences (P>0.05) among the three groups (Table 1).
Discussion
This is the first study comparing two different species of probiotics; we demonstrate their efficacy in the prevention of gastrointestinal colonization by Candida species and in reducing gastrointestinal symptoms; furthermore, we observed a lower incidence of neurological problems in infants supplemented with probiotics.
We selected L. reuteri ATCC 55730 and L. rhamnosus ATCC 53103 because they are the most well-documented probiotics in terms of safety and capability to colonize the human intestine;16, 17, 25, 26, 31 for this reason, no fecal analysis of intestinal colonization was conducted.
The results of this study show a significant reduction in gastrointestinal colonization by Candida among the preterms treated with probiotics, as reported by a previous study.32
The number of invasive Candida infections between the three groups was not statistically different even if the frequency in the control group was higher than in the groups treated with L. reuteri and L. rhamnosus. Wagner et al.33 studied athymic mice with an oral challenge of Candida and reported that fungal colonization was attenuated in mice with a normal gut flora compared with those with germ-free microflora. These data highlight the fact that the intestinal microflora may be as important as an intact immune system in preventing fungal colonization.34, 35, 36, 37
Manzoni et al.,32 in a prospective, randomized, blind, clinical trial involving 80 very-low-birth-weight infants, reported that oral administration of L. rhamnosus reduced the incidence and the intensity of fungal enteric colonization with no associated adverse effects. The authors suggested a fold reduction in fungal gut colonization in newborns with a birth weight <1000 g using a combination of probiotics and drugs (fluconazole in prophylaxis). The potential mechanisms by which probiotics may modify fungal ecology in the gut include a competitive exclusion of fungi, as well as a reduction in their ability to colonize the enteric mucosa through enhanced mucosal IgA responses; it could permit changes in intestinal permeability, with an increased gut mucosal barrier against fungi and the modification of the host response to fungal products.
Our study shows the potential beneficial effects of L. reuteri supplementation on clinical and physiological variables related to gut function, consistently higher than L. rhamnosus and control groups. L. reuteri improves feeding tolerance, bowel habits and gastric motility by increasing the gastric emptying rate and reducing the fasting antral area, and then reducing episodes of regurgitation. Different results between the two probiotics could be due to a lower colonization of L. rhamnosus in preterm infants with a birth weight <1500 g (25%) than in those with a birth weight between 1500 and 1999 g (50%).27 Probiotic colonization depends on the interplay of multiple factors in the intestinal milieu. The first and foremost factor is the bacterial tolerance to both acid and alkaline environments. L. reuteri is reported to be highly resistant to the acid pH and to the antibiotic treatment thanks to a reduced length of the parenteral nutrition and of the use of central venosus catheter with lesser bacterial infection.36, 38 The frequent use of broad-spectrum antibiotics, in preterms alters the intestinal milieu, favoring colonization and infections with multiresistent bacteria and fungi.28 Our results show that probiotic supplementation reduces Candida colonization in NICU newborns, leading to a reduction in the duration of hospitalization.
Another interesting result of our study is that the control group fed a higher percentage of hydrolyzed formula milk than did the probiotic groups, especially the L. reuteri group, which showed an increased food tolerance, probably due to its changes in intestinal flora, improvement in mucosal barrier and anti-inflammatory properties as suggested by other authors.22, 39
Postnatal steroid has been frequently used in the management of infants with chronic lung disease and airway edema hypotension, but their use is not free from adverse effects as the increased risk for the development of fungal sepsis and several retinopaty of prematurity40 or the increased impairment with higher dose.41
The neurological status of preterms with a history of sepsis was examined in few studies, reporting that these infants had a worse neuronal development compared to control group at 18 months of age.18 In a large cohort of 6093 extremely low-birth-weight infants, Stoll et al.42 reported the neurodevelopmental differences between infected and uninfected infants, with an increased rate of adverse effects, as mental and psychomotor retardation, vision and hearing impairment and cerebral palsy, in children with infections. Although our neurological follow-up stopped at 1 year, our results are in accordance with the literature, as a higher incidence of poor neurological outcomes was observed in the control group, showing a higher frequency of Candida infections and stool colonization compared with the probiotic groups. As in the neonatal period, a similar neurological condition was reported (similar number of abnormal cerebral US), we suppose that candidemia may have a significant role in the neurological outcomes of the control group newborns. Therefore, our data suggest that a close neurological follow-up is necessary for infants with a diagnosis of sepsis and that probiotic supplementation may improve their neurological status.
In conclusion, the use of probiotics appears to be effective in the prevention of gastrointestinal colonization by Candida and in the protection from mycotic infections.
Both used probiotics had good safety and did not show any adverse reactions or side effects in preterm infants. Furthermore, they induced an attenuation of gastrointestinal symptoms and a more rapid weaning from the total parenteral nutrition, with a reduction in the number of days of hospitalization.
In the groups of newborns supplemented with probiotics, we noted fewer gastrointestinal symptoms, earlier achievement of food tolerance, a more rapid discontinuation of parenteral nutrition and a better neurological outcome at 1 year, than the control group. Further studies with a larger newborn population weighing <1000 g are required to establish both the safety and the efficacy of probiotics in NICU.
References
Makhoul IR, Kassis I, Smolkin T, Tamir A, Sujov P . Review of 49 neonates with acquired fungal sepsis. Pediatrics 2001; 107: 61–66.
Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B, In Collaboration with the Israel Neonatal Network. Epidemiological, clinical and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 2002; 109: 34–39.
Makhoul IR, Bental Y, Weisbrod M, Sujov P, Lusky A, Reichman B . Candida versus bacterial late-onset sepsis in very low birth weight infants in Israel: a national survey. J Hosp Infect 2007; 65: 237–243.
Gaynes RP, Edwards JR, Jarvis WR, Culver DH, Tolson JS, Martone WJ . Nosocomial infections among neonates in high-risk nurseries in the United States. Pediatrics 1996; 98: 357–361.
Rabalais GP, Samiec TD, Bryant KK, Lewis JJ . Invasive candidiasis in infant weighing more than 2500 grams at birth admitted to a neonatal intensive care unit. Pediatric Infect Dis J 1996; 15: 348–352.
Smith H, Congdon P . Neonatal systemic candidiasis. Arch Dis Child 1985; 60: 365–3695.
Pappu-Katikaneni LD, Rao KP, Banister E . Gastrointestinal colonization with yeast species and Candida septicemia in very low birth weight infants. Mycoses 1990; 33: 20–23.
Gewold IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P . Stool microflora in extremely low birth weight infants. Arch Dis Child Fetal Neonatal Ed 1999; 80: F167–F173.
Kennedy MJ, Volz PA . Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun 1985; 49: 654–663.
Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, National Epidemiology of Mycoses Study Group. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J 2001; 20 (12): 1119–1124.
Tancrède C . Role of human microflora in health and disease. Eur J Clin Microbiol Infect Dis 1992; 11: 1012–1015.
Hammerman C, Bin-Nun A, Kaplan M . Germ warfare: probiotics in defence of premature gut. Clin Perinat 2004; 31: 489–500.
Cummings JH, Macfarlane GT . Colonic microflora: nutrition and health. Nutrition 1997; 13: 476–478.
Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E . Probiotcs in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001; 357: 1076–1079.
Neish AS . The gut microflora and intestinal epithelial cells: a continuing dialogue. Microbes Infect 2002; 4: 309–317.
Millar MR, Bacon C, Smith SL, Walker V, Hall MA . Enteral feeding of premature infants with Lactobacillus GG. Arch Dis Child 1993; 69: 483–487.
Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K . Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 2004; 70 (2): 1176–1181.
Benjamin DK, Stoll BJ, Fanaroff AA, Mc Donald SA, Poole K, Laptook A et al. Neonatal Candidiasis among extremely low birth weight infants: risk factors, mortality rates and neurodevelopment outcomes at 18 to 22 months. Pediatrics 2006; 117: 84–92.
Ziegler EE, Thureen PJ, Carlson SJ . Aggressive nutrition of very low birthweight infant. Clin Perinatol 2002; 29: 225–244.
Tsang RC, Uauy R, Koletzko B, Zlotkin SH . Nutrition of the preterm infant. Scientific Basis and Pratical Guidelines. Digital Educational Publishing: Cincinnati, 2005.
Savino F, Pelle E, Palumeri E, Oggero R, Miniero R . Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 2007; 119 (1): 124–130.
Gray JW . Surveillance of infection in neonatal intensive care units. Early Hum Develop 2007; 83: 157–163.
Oliveri S, Trovato L, Betta P, Romeo MG, Nicoletti G . Experience with the Platelia Candida ELISA for the diagnosis of invasive candidosis in neonatal patients. Clin Microbiol Infect 2008; 14: 377–397 Research Notes 391.
Manzoni P, Pedicino R, Stolfi I, Decembrino L, Castagnola E, Pugni L, et al., the Neonatal Fungal Infections Task Force of the Italian Neonatology Society. Criteri per una corretta Diagnosi delle Infezioni Fungine Sistemiche Neonatali in TIN: i suggerimenti della Task Force per le Infezioni Fungine Neonatali del G.S.I.N. Pediatr Med Chir 2004; 26 (2): 89–95.
Karvonen A, Casas I, Vesikari T . Safety and possible antidiarrhoeal effect of the probiotic Lactobacillus reuteri after oral administration to neonates. Clin Nutr 2001; 20 (suppl 3): 63: abstract 216.
Ramesh A, Nidhi S, Rama C, Ashok D, Vinod KP, Ira GH et al. Effect of oral Lactobacillus GG on Entyeric Microflora in low birth-weight neonates. JPGN 2003; 36: 397.
Pappas PG, Rex JH, Sobel JD, Filler SD, Dismukes WE, Walsh TJ et al. Guidelines for treatment of Candidiasis. Clin Infect Dis 2004; 38: 161.
Kaufman D, Fairchild KD . Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infant. Clin Microbiol Rev 2004; 17: 638–680.
Haataja L, Mercuri E, Regev R, Cowan F, Rutherford M, Dubowitz V et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr 1999; 135: 153–161.
Larroque B, Marret S, Ancel P, Arnaud C, Marpeau L, Supernant K et al. White matter damage and intraventricular hemorrhage in very preterm infants: the epipage study. J Pediatr 2003; 143: 477–483.
Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M . Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 1997; 76: F101–F107.
Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis 2006; 42 (12): 1735–1742. Epub 2006 May.
Wagner RD, Pieron C, Warner T, Dohnalek M, Farmer J, Roberts L et al. Biotherapeutic effect of probiotic bacteria on candidiasis in immunodeficient mice. Infect Immun 1997; 65: 4165–4172.
Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K . Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 2004; 70: 1176–1181.
Cukrowska B, LodInova-ZadnIkova R, Enders C, Sonnenborn U, Schulze J, Tlaskalová-Hogenová H . Specific proliferative and antibody responses of premature infants to intestinal colonization with non-pathogenic probiotic E. coli strain Nissle 1917. Scand J Immunol 2002; 55: 204–209.
Mountzouris KC, McCartney AL, Gibson GR . Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr 2002; 87: 405–420.
Reuman PD, Duckworth DH, Smith KL, Kagan R, Bucciarelli RL, Ayoub EM . Lack of effect of Lactobacillus GG on gastrointestinal bacterial colonization in premature infants. Pediatr Infect Dis J 1986; 5: 663–668.
De Vecchi E, Nicola L, Zanini S, Drago L . In vitro screening of probiotic characteristics of some Italian products. J Chemother 2008; 20 (3): 341–347.
Indrio F, Riezzo G, Raimondi F, Bisceglia M, Cavallo L, Francavilla R . The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J Pediatr 2008; 152 (6): 801–806.
Haroon Parupia MF, Dhanireddy R . Association of postnatal dexamethasone use and fungal sepsis in the development of severe retinopaty of prematurity and progression to laser therapy in extremely low-birth-weight infants. J Perinatol 2001; 21 (4): 242–247.
Wilson-Costello D, Walsh MC, Langer JH, Guillet R, Laptook AR, Stoll BJ, et al., for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 2009; 123: e430–e437.
Stoll B, Hansen N, Adams-Chapman I, Fanaroff A, Hintz S, Vohr B et al. Neurodevelopmental and growth impairnment among extremely low birth weight infants with neonatal infection. JAMA 2004; 292: 2357–2365.
Acknowledgements
We thank Dr S Cilauro, MA Conversano, M Caracciolo, M Marletta, A Saporito, S Castglione and all the nurses of the NICU for their kind collaboration. We also thank Dr A Sciacca of the O.U. Laboratory Analysis, University of Catania, and Dr P Sciacca of the Pediatric Cardiology Unit of the University of Catania for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Romeo, M., Romeo, D., Trovato, L. et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol 31, 63–69 (2011). https://doi.org/10.1038/jp.2010.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2010.57
Keywords
This article is cited by
-
Growth and neuro-developmental outcomes of probiotic supplemented preterm infants—a systematic review and meta-analysis
European Journal of Clinical Nutrition (2023)
-
Bifidobacterium infantis as a probiotic in preterm infants: a systematic review and meta-analysis
Pediatric Research (2023)
-
Effectiveness of a probiotic combination on the neurodevelopment of the very premature infant
Scientific Reports (2023)
-
Neonatal sepsis definitions from randomised clinical trials
Pediatric Research (2023)
-
Is Regular Probiotic Practice Safe for Management of Sepsis?
Chinese Journal of Integrative Medicine (2022)