Abstract
A DASH (dietary approaches to stop hypertension) dietary pattern rich in fruits and vegetables and low-fat dairy products with increased dietary protein provided primarily from plant protein sources decreases blood pressure. No studies, however, have evaluated the effects of a DASH-like diet with increased dietary protein from lean beef on blood pressure and vascular health. The aim of this study was to study the effect of DASH-like diets that provided different amounts of protein from lean beef (DASH 28 g beef per day; beef in an optimal lean diet (BOLD) 113 g beef per day; beef in an optimal lean diet plus additional protein (BOLD+) 153 g beef per day) on blood pressure, endothelial function and vascular reactivity versus a healthy American diet (HAD). Using a randomized, crossover study design, 36 normotensive participants (systolic blood pressure (SBP), 116±3.6 mm Hg) were fed four isocaloric diets,: HAD (33% total fat, 12% saturated fatty acids (SFA), 17% protein (PRO), 20 g beef per day), DASH (27% total fat, 6% SFA, 18% PRO, 28 g beef per day), BOLD (28% total fat, 6% SFA, 19% PRO, 113 g beef per day) and BOLD+ (28% total fat, 6% SFA, 27% PRO, 153 g beef per day), for 5 weeks. SBP decreased (P<0.05) in subjects on the BOLD+ diet (111.4±1.9 mm Hg) versus HAD (115.7±1.9). There were no significant effects of the DASH and BOLD diets on SBP. Augmentation index (AI) was significantly reduced in participants on the BOLD diet (−4.1%). There were no significant effects of the dietary treatments on diastolic blood pressure or endothelial function (as measured by peripheral arterial tonometry). A moderate protein DASH-like diet including lean beef decreased SBP in normotensive individuals. The inclusion of lean beef in a heart healthy diet also reduced peripheral vascular constriction.
Similar content being viewed by others
Introduction
Atherosclerotic cardiovascular disease (CVD) is a multifactorial disease. Estimates indicate that more than 82 million American adults (1 in 3) have one or more types of CVD.1, 2 Many individuals in all ethnic populations have multiple risk factors for CVD, and the number of risk factors in individuals without diagnosed CVD is increasing.1 The major risk factors include smoking status, elevated body weight, total cholesterol, low-density lipoprotein cholesterol, blood pressure (BP) and fasting glucose.1 Approximately 60% of Caucasian adults and 80% of African American adults have at least one risk factor. DASH (dietary approaches to stop hypertension) is the ‘gold standard’ dietary pattern recommended by the American Heart Association,3 American Society of Hypertension2 and 2010 Dietary Guidelines for Americans4 for reducing many of these major CVD risk factors including abnormal lipids and lipoproteins, high BP, overweight/obesity and elevated blood glucose levels.5, 6 The DASH dietary pattern is reduced in saturated fatty acids (SFA), with emphasis on dietary carbohydrate from fruits, vegetables and whole grains; multiple minerals (potassium, magnesium and calcium) and fibre are also increased.
In addition to the major risk factors for CVD, there are other risk factors including those related to vascular health (endothelial function, vascular reactivity and so on). Our understanding of how diet affects vascular health is still evolving and more information is needed.
Individuals are often advised to avoid or restrict beef because it is a source of saturated fat in the diet. However, many Americans enjoy beef, commonly choosing cuts deemed lean by United States Department of Agriculture (USDA), and report better adherence to dietary advice that includes some lean beef.7 In addition, beef’s contribution to SFA in the American diet is often overstated in that it is not one of the top five contributors of SFA for Americans.4 In the BOLD Study, we showed that the inclusion of lean beef (4.0 or 5.4 oz per day) in a DASH-like diet decreased total cholesterol and low-density lipoprotein cholesterol similarly to the DASH diet.8 The DASH diet guidelines suggest reducing red meat as a strategy for controlling saturated fat;9 however, little is known about the effects on vascular health when lean beef is incorporated in a DASH diet.
In the present study conducted with normotensive individuals, we evaluated the effects of a traditional DASH diet as well as a DASH-like diet containing lean beef (beef in an optimal lean diet (BOLD); 113 g beef per day) and a moderate protein diet containing lean beef (BOLD+; 153 g beef per day) compared with a healthy American diet (HAD) as the control on vascular health, a secondary end point in the BOLD Study.
Subjects and methods
Subjects
The methods used for this study have been described in detail previously.8 Nonsmoking normo- or pre-hypertensive (BP <140/90 mm Hg) men and women (30–65 years) with moderately elevated low-density lipoprotein cholesterol (110–176 mg dl−1) were recruited.8 Additional inclusion criteria were body mass index (18.5–37 kg m−2) and fasting triglycerides <350 mg dl−1. Participants taking prescribed BP-lowering medication were eligible as long as their BP was below the exclusion criteria (one participant on BP medication was enrolled in the trial but excluded from the vascular health analyses). Exclusion criteria were: established CVD, stroke, diabetes, liver, kidney or autoimmune disease, the use of cholesterol/lipid-lowering medication or supplements (psyllium, fish oil, soy lecithin and phytoestrogens), being pregnant or lactating, experiencing weight loss of ⩾10% of body weight within the 6 months before enrolling in the study and vegetarianism. The Institutional Review Board at The Pennsylvania State University approved the experimental protocol, and all subjects provided written informed consent. This study is registered at ClinicalTrials.gov NCT00937898.
Study design
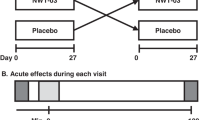
The study employed a four-period, randomized, crossover, controlled-feeding design. Subjects were randomly assigned to a treatment (diet) order, and consumed each diet (HAD, DASH, BOLD and BOLD+) for 5 weeks. The diet periods were separated by a brief compliance break (average 1 week). On two consecutive days at the beginning of the study (baseline) and at the end of each diet period, participants completed a series of clinical and physical assessments (blood draw, height and weight) at the General Clinical Research Center of The Pennsylvania State University. The initial participants were enrolled in the study in September 2007; the final participants completed the study in March 2009.
Diets
The composition of the experimental diets is presented in Table 1. The Harris–Benedict equation was used to estimate each participant’s energy needs, participants were monitored (daily weigh-ins) to verify they remained weight stable and calorie adjustments were made in 100 kcal increment to assure that weight remained stable for the duration of the study. All diets were rich in fruits, vegetables and lean meats consistent with food-based dietary recommendations. The three experimental diets (DASH, BOLD and BOLD+) contained similar amounts of total fat, SFA, monounsaturated fatty acids, polyunsaturated fatty acids and cholesterol. The HAD was higher in total fat, SFA, monounsaturated fatty acids, polyunsaturated fatty acids and cholesterol, and was lower in total fibre. The BOLD and DASH diets were matched for macronutrient composition. The BOLD+ diet was higher in protein (27% of total energy; 19% plant, 26% dairy, 42% lean beef and 12% other animal sources) as compared with the HAD (17%; 13% plant, 26% dairy, 12% lean beef and 49% other animal sources), DASH (18%; 20% plant, 31% dairy, 9% lean beef and 40% other animal sources) and BOLD (19%; 13% plant, 23% dairy, 53% lean beef and 11% other animal sources) diets, and lower in carbohydrate (45 vs 50–54%) (Table 1). A description of the food groups (and respective servings) fed has been published previously.8
Although matched for protein, the BOLD and DASH diets differed in the quantity of lean beef (Table 1). Select grade top round, chuck shoulder pot roast and 95% lean ground beef were used in the study. The meat was prepared via braising, grilling or frying (95% lean ground beef only), and was never cooked over an open flame in order to prevent charring.
A 6-day menu cycle was used throughout the study (1800–3600 kcal per day). All meals and snacks were prepared at the Metabolic Diet Study Center at Pennsylvania State University. Participants ate one meal per day (Monday–Friday) in the Metabolic Diet Study Center and their other meals were prepared and packed for off-site consumption. On weekends when the Study Center was closed, participants received a cooler that contained all of their meals and snacks for 2 days. Compliance with the prepared diets was monitored via self-report to document whether study foods/meals were omitted and/or replaced. Participants limited caffeinated beverages to 8 oz per day and alcoholic beverages to <2 servings per week. Participants were allowed to continue their current exercise regimen but were instructed not to increase or decrease duration or intensity during the study.
Clinical assessments
Body weight was measured each weekday in the Metabolic Diet Study Center before eating that day’s meal and at each laboratory visit. Blood samples were collected after a 10–12-h fast. Serum and plasma aliquots were stored at −80 °C until time of analysis.
Vascular health
Measures of vascular health were secondary end points in the BOLD Study. BP was assessed using a single measurement at the beginning of the study and at the end of each diet period before the baseline period of the endothelial function test (Dinamap Pro 100, Critikon, Milwaukee, WI, USA). Participants were seated with arm at heart level and appropriate cuff sizes were used. After a 12-h fast, EndoPAT2000 (Itamar Medical, Ltd, Caesarea, Israel) was used to measure relative changes in pulse wave amplitude before vs after occlusion.10 The EndoPAT technique is validated as a measure of endothelial function.10, 11 Two flexible probes were placed on the index fingers of the right (ischaemic) and left (control) hands, and a counter pressure was applied to both fingers continuously throughout the test. A BP cuff was placed on the right forearm and pulse amplitude was measured during baseline (5 min), occlusion (5 min) and reactive hyperaemia (5 min). Reactive hyperaemia index (RHI) was calculated as the ratio of the average pulse wave amplitude during hyperaemia (60 to 120 s of the post-occlusion period) to the average pulse wave amplitude during baseline in the occluded hand divided by the same values in the control hand and then multiplied by a baseline correction factor. We also calculated the Framingham RHI (FRHI) as described previously.12, 13
The EndoPAT device was used to generate the augmentation index (AI). The EndoPAT-generated AI measurement is determined from the baseline resting pulse wave. In stiff arteries, the pulse wave travels rapidly to the periphery where it encounters resistance at the peripheral arterioles, and the reflected wave augments central BP. Thus, higher AI indicates greater arterial stiffness. Proprietary software automatically identifies inflection points distinguishing the systolic peak and the reflected peak for the calculation of this ratio and converts it into a percentage (p1−p2/p1 × 100).14 EndoPAT-derived AI measures correlate well with AI measures from other devices.15 AI can be adjusted to a heart rate of 75 beats per min to correct for the independent effect of heart rate on AI measurement; both AI and AI at 75 beats per min are reported.
Statistical analysis
All statistical analyses were performed using SAS (Version 9.2; Statistical Analyses System, Cary, NC, USA). Two sample t-tests were used to determine significant differences between genders at baseline for each outcome variable. The residuals for each variable were used to assess normality. Logarithmic transformations were used for non-normally distributed variables (AI and RHI). The mixed models procedure (PROC MIXED) was used to test the effects of diet and order on the outcome variables. A repeated analysis of covariance (repeated for diet) was used with age, weight and baseline values as covariates. Tukey–Kramer adjusted P-values were used to determine whether the differences between the diets for outcome variables were significant (P<0.05).
Results
Figure 1 presents information about the number of subjects who responded to the study advertisements (n=968); completed the phone interview/screening (n=171); completed the clinical screening (n=86); and enrolled in the study (n=42). The vast majority of potential subjects were excluded based on the inclusion and exclusion criteria of the study after they completed the screening processes. Some individuals who met the study eligibility criteria elected to not participate because of the requirements imposed by the study. During the study, one subject dropped out because of a job change and relocation, one to an unrelated illness and four because of an inability to adhere to the dietary protocol (the latter occurred within the first week of the study). One participant was on BP-lowering medication for the duration of the study and was excluded in the analysis. There were 36 subjects included in the final analysis (Figure 1).
Baseline subject characteristics are presented in Table 2. Females had a significantly higher FRHI (0.79 vs 0.43; P=0.04) compared with males. Males had a significantly greater body mass index (27.3 vs 24.8 kg m−2; P=0.02) compared with females (Table 2). There were no gender differences in response to any of the dietary treatments. Subject adherence to the prescribed diets was 93% according to daily self-reporting forms. Body weight was maintained during the diet periods within ±2.2 kg. The metabolic status (lipids, glucose, insulin and C-reactive protein) of these subjects has been described previously.8
Blood pressure
Systolic blood pressure (SBP) was significantly reduced following the BOLD+ diet compared with the HAD (P<0.01); no other significant reductions in SBP were observed. There were no significant changes in DBP on the DASH, BOLD or BOLD+ diets.
Endothelial function and vascular stiffness
The RHI and FRHI scores did not differ among diets (Table 3). AI significantly decreased following the BOLD diet compared with the HAD, DASH and BOLD+ diets (Table 3).
A significant interaction was observed between subject age and diet for AI. To further explore this interaction, subjects were grouped according to whether their age was a risk factor for CVD (females ⩾55 years and males ⩾45 years).16 This secondary analysis revealed that AI was significantly reduced on the BOLD diet in younger participants, but not older individuals.
Discussion
The BOLD Study is the first controlled clinical trial to show that a moderate protein diet (based on the DASH eating plan) that emphasized lean beef (113 g per day) as the main protein source reduced SBP in normotensive individuals when compared with a healthy control diet that was lower in protein and higher in carbohydrate and saturated fat. The BOLD+ diet contained 10% more calories from protein and reduced SBP by 4.2 mm Hg versus HAD, whereas the BOLD and DASH diet elicited nonsignificant reductions of 1.6 and 2.8 mm Hg, respectively.
In the OmniHeart trial, the high-protein diet that had a comparable macronutrient profile to the BOLD+ diet resulted in a −9.5 mm Hg reduction in SBP from baseline.17 The difference in the magnitude of BP reductions in the present study and the protein diet evaluated in the OmniHeart trial could be due, in part, to the participants studied. Specifically, participants in the BOLD Study were normotensive (mean baseline SBP, 116±3.6 mm Hg), whereas participants in the OmniHeart trial were pre-hypertensive (mean baseline SBP, 131.3±10.8 mm Hg). In the BOLD+ diet, as in the higher protein OmniHeart diet, increases in total protein (from either animal or plant protein) suggest that the BP reductions reflect a total protein effect or the synchronous reduction in carbohydrates.
Compared with the original DASH trial (which lowered SBP by −3.5 mm Hg),18 the BOLD and DASH diets yielded similar, yet nonsignificant changes in SBP versus HAD (−1.9 and −2.8 mm Hg, respectively). The minor differences in response to the DASH diet and the similarly designed BOLD diet in our study may be because of the normotensive status of the study population compared with the pre-hypertensive/hypertensive participants in the DASH trial. Individuals with hypertension have greater reductions in BP following a heart healthy diet,19 as well as after weight loss20 compared with their normotensive counterparts. In addition, our study had far fewer subjects (n=36) than the DASH trial (n=459), and this could explain the lack of statistical significance for the SBP change observed. The differences in the response observed in the BOLD Study may also be due in part to differences in total and saturated fat between the control diet used in the initial DASH study (total fat=37%; SFA=16% of total energy) and the HAD (total fat =33%; SFA=12% of total energy).21
In addition to the aforementioned protein effect (or carbohydrate reduction), potassium, magnesium, sodium and calcium are minerals of importance with respect to their role in modulating BP.22 Sodium and calcium intakes were similar for the HAD and BOLD+ diets (Table 1). Potassium and magnesium levels were lower in the HAD compared with the BOLD+ diet. The BOLD+ diet provided similar amounts of potassium and magnesium compared with the original DASH study (4415 and 480 mg per day, respectively),18 and potassium levels were similar to those recommended by the American Society of Hypertension (4700 mg per day).2 A 2006 Cochrane review on magnesium supplementation for the treatment of high BP did not find evidence to support a causal relationship.23 A systematic review by Dickinson et al.24 found that magnesium supplementation (0.2–1.0 g per day) reduced SBP by 1.3 mm Hg, but this was nonsignificant. Therefore, we believe that it is unlikely that the 121 mg per day difference in magnesium between the HAD and BOLD+ diet significantly contributed to the reduction in SBP. We hasten to add, however, that in a diet that is also increased in other nutrients that are shown to lower BP, a small increase in magnesium may contribute to a BP-lowering effect.
It is also unlikely that dietary fibre is responsible for the reduction in SBP. A meta-analysis of 25 randomized controlled trials found no effect of dietary fibre intake on SBP in normotensive individuals.25 Thus, the 14 g per day increase in dietary fibre between the HAD and BOLD+ diet likely did not influence SBP in normotensive subjects in the BOLD Study. Although the separate effect of fibre and select minerals does not fully explain the BP-lowering effects, there is most likely an effect of the synergy of these changes, as seen in the original DASH trial. Taking this into account, we still propose that the primary explanation for the changes in SBP in our normotensive study population was most likely because of the increase in total protein (from a variety of protein sources, including lean beef) that also led to a slight decrease in carbohydrate. Further studies are needed to resolve this question.
On the BOLD diet, AI was significantly reduced compared with the HAD, despite no significant changes in DBP or SBP. In addition, AI was not significantly correlated with DBP (R=0.063, P=0.4) or SBP (R=0.15, P=0.06). However, there is conflicting evidence regarding the relationship between peripheral BP and AI.26, 27, 28 Hamburg et al.13 hypothesized that the discrepancy in the relationship between peripheral arterial tonometry measures, like AI, and systemic BP may be because of the limited effect of systemic BP on the distal microcirculation. This might be the case in the BOLD Study as significant improvements in AI were observed in the BOLD but not BOLD+ diets, whereas SBP was reduced in the BOLD+ but not BOLD or DASH diets.
AI and age are also related.29 Our results are consistent with this finding as a secondary analysis revealed a significant reduction in AI following the BOLD diet only in younger participants (males <45 years and females <55 years). This suggests that the arterial stiffness associated with ageing30 was not modified by the BOLD diet. Thus, dietary interventions designed to improve vascular reactivity may need to be initiated earlier in life to have a significant effect.
The mechanism accounting for the significant reduction in AI following the BOLD diet compared with the HAD, DAS and BOLD+ diets is unclear. This is one of the first controlled-feeding studies to measure the impact of different macronutrient composition (as well as protein sources) on AI. The improvements in AI observed for the BOLD diet underscore the need to better understand the effects of diet and protein (that is, quantity and source) on vascular elasticity.
The endothelial function results of the current study (as assessed by EndoPat) agree with those of a 35-day controlled-feeding intervention conducted by Vega-López et al.31 who found no effect of increasing the dietary lysine/arginine ratio (0.7 to 1.4; a common measure of the animal/plant protein ratio) of a low SFA diet (<7% total calories) on flow-mediated dilation or peripheral arterial tonometry (precursor to RHI measurement provided by EndoPat 2000). Al-Solaiman et al.32 and Hodson et al.33 also found no changes in endothelial function in healthy individuals following the DASH diet.
There were three potential limitations of the vascular health end points in the BOLD Study. One potential limitation was that BP was only measured once at baseline and at each end point visit. A minimum of two measurements taken 1 min apart is the preferred method to reduce measurement error. In addition, the null finding for RHI and FRHI may have been influenced by the menstrual phase that was not controlled for in the present study; however, we also did not observe any significant changes in RHI or FRHI in male subjects. Finally, in this study, macronutrient intakes and other nutrient guidelines were set based on percent of total calories or in the context of the base diet of 2100 calories. Although the calorie levels for individuals were adjusted up or down based on the energy needs required to maintain a participant’s weight, nutrients such as fibre, potassium, sodium and magnesium were also increased or decreased but not always in an exact proportional manner. Because it is not known whether meeting the exact nutrient targets established in the DASH study for different calorie levels is important, this could be one possible reason why a statistically significant BP reduction was not observed for the DASH and BOLD diets in the present study.
The BOLD diet was the only diet that significantly reduced AI, and the BOLD+ diet was the only diet that significantly reduced SBP. These findings suggest that heart-healthy diets containing different amounts of macronutrients including those contained in lean beef (that is, primarily protein) can positively affect vascular health, although via potentially different mechanisms. It has been suggested previously that increased dietary plant protein (versus animal protein) may be responsible for the protein-associated reductions in BP.34 However, the present findings show that a variety of protein sources including lean beef can also be used to increase total dietary protein in a heart-healthy diet as a strategy to reduce SBP in normotensive individuals. Thus, increasing total dietary protein (or decreasing dietary carbohydrate) in combination with a diet rich in fruits, vegetables, fibre and low-fat dairy appears to play an important role in reducing SBP.
We had previously shown8 that DASH, BOLD and BOLD+ each lowered cholesterol similarly compared with HAD and that these diets had no effect on fasting glucose and insulin levels. Thus, it is unlikely that the differential effects of DASH, BOLD and BOLD+ on vascular status are mediated by changes in lipids, glucose or insulin.
Further controlled clinical trials are needed to elucidate the role and mechanism(s) of action of both protein sources and quantity on BP and vascular health in normotensive and hypertensive individuals.

References
Roger VL . Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123 (4): e18–e209.
Appel L, Giles T, Black H, Izzo J, Materson B, Oparil S et al. ASH Position Paper: dietary approaches to lower blood pressure. J Clin Hypertens 2009; 11 (7): 358–368.
Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007; 115 (21): 2761–2788.
2010 Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Washington, DC: U.S. Government Printing Office.
Hozawa A, Folsom AR, Sharrett AR, Chambless LE . Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects—Atherosclerosis Risk in Communities Study. Arch Intern Med 2007; 167 (6): 573–579.
Unal B, Critchley JA, Capewell S . Modelling the decline in coronary heart disease deaths in England and Wales, 1981–2000: comparing contributions from primary prevention and secondary prevention. BMJ 2005; 331 (7517): 614–620.
Davidson MH, Hunninghake D, Maki KC, Kwiterovich PO Jr., Kafonek S . Comparison of the effects of lean red meat vs lean white meat on serum lipid levels among free-living persons with hypercholesterolemia: a long-term, randomized clinical trial. Arch Intern Med 1999; 159 (12): 1331–1338.
Roussell MA, Hill AM, Gaugler TL, West SG, Vanden Heuvel JP, Alaupovic P et al. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Amer J Clin Nutr 2012; 95 (1): 9–13.
Vogt TM, Appel LJ, Obarzanek E, Moore TJ, Vollmer WM, Svetkey LP et al. Dietary Approaches to Stop Hypertension: rationale, design, and methods. J Am Diet Assoc 1999; 99 (8 Suppl 1): S12–S18.
Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr., Kuvin JT, Lerman A . Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004; 44 (11): 2137–2141.
Research Highlights–editorial review of Bonetti PO et al.. A noninvasive test for endothelial dysfunction. Nat Clin Pract Cardiovasc Med 2005; 2 (2): 64–65.
Hamburg NM, Benjamin EJ . Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 2009; 19 (1): 6–11.
Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008; 117 (19): 2467–2474.
Heffernan KS, Patvardhan EA, Hession M, Ruan J, Karas RH, Kuvin JT . Elevated augmentation index derived from peripheral arterial tonometry is associated with abnormal ventricular–vascular coupling. Clin Physiol Funct Imaging 2010; 30 (5): 313–317.
Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care 2004; 27 (12): 2911–2917.
Adult Treatment Panel III Final Report. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Circulation 2002; 106 (25): 3143–3421.
Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER III et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart Randomized Trial. JAMA 2005; 294 (19): 2455–2464.
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM et al. A clinical trial of the effects of dietary patterns on blood pressure. New Engl J Med 1997; 336 (16): 1117–1124.
Resnick LM, Oparil S, Chait A, Haynes RB, Kris-Etherton P, Stern JS et al. Factors affecting blood pressure responses to diet: the Vanguard study. Am J Hypertens 2000; 13 (9): 956–965.
Leiter LA, Abbott D, Campbell NR, Mendelson R, Ogilvie RI, Chockalingam A . Lifestyle modifications to prevent and control hypertension. 2. Recommendations on obesity and weight loss. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ 1999; 160 (9 Suppl): S7–12.
Rasmussen BM, Vessby B, Uusitupa M, Berglund L, Pedersen E, Riccardi G et al. Effects of dietary saturated, monounsaturated, and n−3 fatty acids on blood pressure in healthy subjects. Am J Clin Nutr 2006; 83 (2): 221–226.
Champagne CM . Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: a review. Nutr Clin Pract 2008; 23 (2): 142–151.
Dickinson HO, Nicolson D, Campbell F, Cook JV, Beyer FR, Ford GA et al. Magnesium Supplementation for the Management of Primary Hypertension in Adults. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2006.
Dickinson H, Mason J, Nicolson D, Campbell F, Beyer F, Cook J et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens 2006; 24 (2): 215–233.
Whelton S, Hyre A, Pedersen B, Yi Y, Whelton P, He J . Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens 2005; 23 (3): 475–481.
Nurnberger J, Dammer S, Opazo Saez A, Philipp T, Schafers RF . Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens 2003; 17 (3): 153–158.
Nakae I, Matsuo S, Matsumoto T, Mitsunami K, Horie M . Augmentation index and pulse wave velocity as indicators of cardiovascular stiffness. Angiology 2008; 59 (4): 421–426.
Takenaka T, Mimura T, Kanno Y, Suzuki H . Qualification of arterial stiffness as a risk factor to the progression of chronic kidney diseases. Am J Nephrol 2005; 25 (5): 417–424.
Kohara K, Tabara Y, Oshiumi A, Miyawaki Y, Kobayashi T, Miki T . Radial augmentation index: a useful and easily obtainable parameter for vascular aging. Am J Hypertens 2005; 18 (1): 11S–14S.
Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004; 43 (6): 1239–1245.
Vega-Lopez S, Matthan NR, Ausman LM, Harding SV, Rideout TC, Ai M et al. Altering dietary lysine:arginine ratio has little effect on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults. Atherosclerosis 2010; 210 (2): 555–562.
Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM . DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. J Hum Hypertens 2010; 24 (4): 237–246.
Hodson L, Harnden KE, Roberts R, Dennis AL, Frayn KN . Does the DASH diet lower blood pressure by altering peripheral vascular function? J Hum Hypertens 2010; 24 (5): 312–319.
Appel LJ . The effects of protein intake on blood pressure and cardiovascular disease. Curr Opin Lipid 2003; 14 (1): 55–59.
Acknowledgements
We thank our study participants for their participation in and commitment to the BOLD Study. Many members of the Kris-Etherton Lab participated in the implementation of the study, including Deborah Bagshaw, Jennifer Fleming, Amy Cifelli, Melissa Hendricks and Marcella Smith. We are also grateful to the nursing and clinical staff of the General Clinical Research Center of The Pennsylvania State University. This study was funded by The Beef Checkoff, and supported by the General Clinical Research Center, Pennsylvania State University (NIH Grant M01RR10732).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PMK-E received travel funds and an honorarium from the Beef Checkoff Program for giving presentations on this research. MAR received travel funds and honoraria from the Beef Checkoff Program for giving presentations on this research. PMK-E, SGW and JPVH received grant funds from the Beef Checkoff Program. The other authors declare no conflict of interest.
Additional information
Author contributions
PMK-E, JPVH, SGW, JSU and PJG designed the research; MAR and AMH conducted the research; MAR and TLG performed the statistical analyses; and MAR, AMH, SGW, JPVH, PJG and PMK-E wrote the manuscript. MAR and PMK-E had primary responsibility for final content. All authors have read and approved the final manuscript.
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Roussell, M., Hill, A., Gaugler, T. et al. Effects of a DASH-like diet containing lean beef on vascular health. J Hum Hypertens 28, 600–605 (2014). https://doi.org/10.1038/jhh.2014.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2014.34
This article is cited by
-
Healthy U.S.-style dietary patterns can be modified to provide increased energy from protein
Nutrition Journal (2022)
-
Red meat and dietary iron intakes are associated with some components of metabolic syndrome: Tehran Lipid and Glucose Study
Journal of Translational Medicine (2019)
-
Red meat and chicken consumption and its association with high blood pressure and obesity in South Korean children and adolescents: a cross-sectional analysis of KSHES, 2011–2015
Nutrition Journal (2017)