Abstract
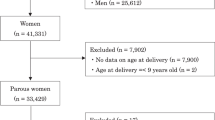
Hypertensive disorder of pregnancy (HDP) is considered an important determinant in the prediction of future hypertension. The aim of this study is to examine whether HDP improves prediction of future hypertension, over prediction based on established risk factors measured during pregnancy. We used a community based cohort study of 2117 women who received antenatal care at a major hospital in Brisbane between 1981 and 1983 and had blood pressure assessed 21 years after the index pregnancy. Of these 2117 women, 193 (9.0%) experienced HDP and 345 (16.3%) had hypertension at 21 years postpartum. For women with HDP, the odds of being hypertensive at 21 years postpartum were 2.46 (95% CI 1.70, 3.56), adjusted for established risk factors including age, education, race, alcohol, cigarettes, exercise and body mass index. Addition of HDP did not improve the prediction model that included these established risk factors, with the area under the curve of receiver operator (AUROC) increasing from 0.710 to 0.716 (P-value for difference in AUROC=0.185). Our findings suggest that HDP is strongly and independently associated with future hypertension, and women who experience this condition should be counselled regarding lifestyle modification and careful ongoing blood pressure monitoring. However, the development of HDP during pregnancy does not improve our capacity to predict future hypertension, over risk factors identifiable at the time of pregnancy. This suggests that counseling regarding lifestyle modification and ongoing blood pressure monitoring might reasonably be provided to all pregnant and postpartum women with identifiable risk factors for future hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Chesley LC . Remote prognosis after eclampsia. Perspect Nephrol Hypertens 1976; 5: 31–40.
Fisher KA, Luger A, Spargo BH, Lindheimer MD . Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore) 1981; 60 (4): 267–276.
Adams EM, Macgillivray I . Long-term effect of preeclampsia on blood-pressure. Lancet 1961; 2: 1373–1375.
Epstein FH . Late vascular effects of toxemia of pregnancy. N Engl J Med 1964; 271: 391–395.
Bellamy L, Casas JP, Hingorani AD, Williams DJ . Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335 (7627): 974.
Kaaja RJ, Greer IA . Manifestations of chronic disease during pregnancy. JAMA 2005; 294 (21): 2751–2757.
Sattar N, Greer IA . Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 2002; 325 (7356): 157–160.
Nakanishi N, Nakamura K, Ichikawa S, Suzuki K, Kawashimo H, Tatara K . Risk factors for the development of hypertension: a 6-year longitudinal study of middle-aged Japanese men. J Hypertens 1998; 16 (6): 753–759.
Skarfors ET, Lithell HO, Selinus I . Risk factors for the development of hypertension: a 10-year longitudinal study in middle-aged men. J Hypertens 1991; 9 (3): 217–223.
Grandi AM, Gaudio G, Fachinetti A, Bianchi L, Nardo B, Zanzi P et al. Hyperinsulinemia, family history of hypertension, and essential hypertension. Am J Hypertens 1996; 9 (8): 732–738.
Callaway LK, David McIntyre H, Williams GM, Najman JM, Lawlor DA, Mamun A et al. Diagnosis and treatment of hypertension 21 years after a hypertensive disorder of pregnancy. Aust N Z J Obstet Gynaecol 2011; 51: 437–440.
Keeping JD, Najman JM, Morrison J, Western JS, Andersen MJ, Williams GM . A prospective longitudinal study of social, psychological and obstetric factors in pregnancy: response rates and demographic characteristics of the 8556 respondents. Br J Obstet Gynaecol 1989; 96 (3): 289–297.
Najman JM, Bor W, O’Callaghan M, Williams GM, Aird R, Shuttlewood G . Cohort profile: The Mater-University of Queensland Study of Pregnancy (MUSP). Int J Epidemiol 2005; 34 (5): 992–997.
Alati R, O’Callaghan M, Najman JM, Williams GM, Bor W, Lawlor DA . Asthma and internalizing behavior problems in adolescence: a longitudinal study. Psychosom Med 2005; 67 (3): 462–470.
Davey DA, MacGillivray I . The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol 1988; 158 (4): 892–898.
Bedford A, Foulds GA . Delusions Symptoms States Inventory: State of Anxiety and Depression (Manual). NFER Publishing: Berkshire, England, 1978.
Rubino A, Pezzarossa B, Zanna V, Ciani N . Fould’s Hierarchy: validation of predictors in psychiatric and dermatological patients. Br J Med Psychol 1997; 70: 395–402.
Najman JM, Andersen MJ, Bor W, O’Callaghan MJ, Williams GM . Postnatal depression-myth and reality: maternal depression before and after the birth of a child. Soc Psychiatry Psychiatr Epidemiol 2000; 35 (1): 19–27.
Justice AC, Covinsky KE, Berlin JA . Assessing the generalizability of prognostic information. Ann Intern Med 1999; 130 (6): 515–524.
Irgens HU, Reisaeter L, Irgens LM, Lie RT . Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ 2001; 323 (7323): 1213–1217.
Garovic VD, Bailey KR, Boerwinkle E, Hunt SC, Weder AB, Curb D et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens 2010; 28 (4): 826–833.
Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L et al. Long-term mortality after preeclampsia. Epidemiology 2005; 16 (2): 206–215.
Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N et al. Associations of pregnancy complications with calculated CVD risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 2012; 125: 1367–1380.
Samwiil L, Mercer C, Jarrett P, O’Malley S and Genetics of Pre-Eclampsia Collaborative Study (GOPEC) Research Midwives. Blood pressure and urinalysis are often omitted in women who have suffered pre-eclampsia at their six-week postnatal check. BJOG 2004; 111 (6): 623–625.
Callaway LK, David McIntyre H, Williams GM, Najman JM, Lawlor DA, Mamun A . Diagnosis and treatment of hypertension 21 years after a hypertensive disorder of pregnancy. Aust N Z J Obstet Gynaecol 2011; 51 (5): 437–440.
Samuels-Kalow ME, Funai EF, Buhimschi C, Norwitz E, Perrin M, Calderon-Margalit R et al. Prepregnancy body mass index, hypertensive disorders of pregnancy, and long-term maternal mortality. Am J Obstet Gynecol 2007; 197 (5): 490.e1–490.e6.
Laivuori H, Tikkanen MJ, Ylikorkala O . Hyperinsulinemia 17 years after preeclamptic first pregnancy. J Clin Endocrinol Metab 1996; 81 (8): 2908–2911.
Pouta A, Hartikainen AL, Sovio U, Gissler M, Laitinen J, McCarthy MI et al. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension 2004; 43 (4): 825–831.
Forest JC, Girouard J, Massé J, Moutquin JM, Kharfi A, Ness RB et al. Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet Gynecol 2005; 105 (6): 1373–1380.
Callaway LK, Prins JB, Chang AM, McIntyre HD . The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust 2006; 184 (2): 56–59.
Acknowledgements
We thank the MUSP Team, MUSP participants, the Mater Misericordiae Hospital and the Schools of Social Science, Population Health, and Medicine at the University of Queensland for their support; and the National Health and Medical Research Council (NHMRC), Queensland Health, the Centre for Accident Research and Road Safety—Queensland (CARRS-Q), the Australian Institute of Criminology (AIC) and National Heart Foundation for funding this project. We wish to specifically thank members of the MUSP-21-Year Follow-up team including Rosemary Aird, Stacey Allerton, Ruth Armstrong, Samantha Batchelor, Pauline Bonnici, Rachael Bor, Emma Brown, Justine Butcher, Fiona Cameron, Narelle Constantine, Sophie Gudgeon, Jatinder Kaur, Jane Maclean, Amanda Margerison, Kobie Mulligan, Kelly Quinlan, Marie Seeman and Jennifer Winn. DAL works in a Centre that receives funding from the UK Medical Research Council (G0600705) and University of Bristol. A Al Mamun is funded by the NHMRC Career Development Awards in Population Health (ID 519756). The core MUSP study was funded by the National Health and Medical Research Council (NHMRC) of Australia.
Disclaimer
The views expressed in this study are those of the authors and not necessarily any funding body. The authors had full access to all data and no funding bodies influenced the analysis or interpretation of results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Callaway, L., Mamun, A., McIntyre, H. et al. Does a history of hypertensive disorders of pregnancy help predict future essential hypertension? Findings from a prospective pregnancy cohort study. J Hum Hypertens 27, 309–314 (2013). https://doi.org/10.1038/jhh.2012.45
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2012.45
Keywords
This article is cited by
-
Association between hypertensive disorders of pregnancy and the risk of postpartum hypertension: a cohort study in women with gestational diabetes
Journal of Human Hypertension (2017)
-
The association between perception of health during pregnancy and the risk of cardiovascular disease: a prospective study
SpringerPlus (2016)