Abstract
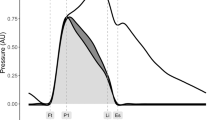
The effects of pressure wave reflection have been incompletely described by the central augmentation index (cAI) and augmented pressure (Pa). We therefore investigated the determinants of amplitude of the reflected wave (Pb), which is independent of the reflected wave transit time (RWTT) and has been shown to predict cardiovascular mortality in the general population. A total of 180 (117 men, mean age 68 years old) patients were recruited. Carotid pressure waveforms derived by tonometry at baseline and 3 min after administration of sublingual nitroglycerin (NTG) were calibrated and then decomposed into the forward and backward waves to yield Pb. The ratio of pre-ejection period/ejection time (PEP/ET) was measured. By stepwise multivariate analysis, independent determinants of Pb included brachial mean blood pressure (β=0.56, P<0.001), heart rate (β=−0.29, P<0.001), age (β=0.20, P<0.001), PEP/ET (β=−0.16, P=0.004) and height (β=−0.13, P=0.018). RWTT, body mass index and sex were significant independent determinants of Pa and cAI but did not contribute to Pb. Change of Pb but not Pa or cAI significantly predicted the changes of carotid systolic (r=0.550, P<0.001) and pulse pressure (r=0.618, P<0.001) after NTG. In conclusion, determinants of Pb differ from those of cAI and Pa. Pb is independent of sex and RWTT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD et al. Role of pulse pressure amplification in arterial hypertension. Experts’ opinion and review of the data. Hypertension 2009; 54: 375–383.
Hope SA, Tay DB, Meredith IT, Cameron JD . Waveform dispersion, not reflection, may be the major determinant of aortic pressure wave morphology. Am J Physiol Heart Circ Physiol 2005; 289: H2497–H2502.
Shimizu M, Kario K . Role of the augmentation index in hypertension. Ther Adv Cardiovasc Dis 2008; 2: 25–35.
Namasivayam M, McDonnell BJ, McEniery CM, O’Rourke MF . Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension 2009; 53: 979–985.
Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JM, Ogilvie RI et al. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension 2008; 51: 105–111.
Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA et al. Wave reflection and arterial stiffness in the prediction of 15-Year all-cause and cardiovascular mortalities. a community-based study. Hypertension 2010; 55: 799–805.
McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR . Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005; 46: 1753–1760.
Segers P, Rietzschel ER, De Buyzere ML, De Bacquer D, Van Bortel LM, De Backer G et al. Assessment of pressure wave reflection: getting the timing right!. Physiol Meas 2007; 28: 1045–1056.
Davies JE, Baksi J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH et al. The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol 2010; 298: H580–H586.
Sakurai M, Yamakado T, Kurachi H, Kato T, Kuroda K, Ishisu R et al. The relationship between aortic augmentation index and pulse wave velocity: an invasive study. J Hypertens 2007; 25: 391–397.
Vyas M, Izzo Jr JL, Lacourciere Y, Arnold JM, Dunlap ME, Amato JL et al. Augmentation index and central aortic stiffness in middle-aged to elderly individuals. Am J Hypertens 2007; 20: 642–647.
Chen CH, Ting CT, Lin SJ, Hsu TL, Yin FCP, Siu CO et al. Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension 1995; 25: 1034–1041.
Mahmud A, Feely J . Beta-blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens 2008; 21: 663–667.
Fitchett DH, Simkus GJ, Beaudry JP, Marpole DGF . Reflected waves in the ascending aorta: effect of glyceryl trinitrate. Cardiovasc Res 1988; 22: 494–500.
Panca AL, Wallenhaupt SL, Kon ND, Tucker WY . Does radial artery pressure accurately reflect aortic pressure? Chest 1992; 102: 1193–1198.
Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25: 359–364.
Yu WC, Chuang SY, Lin YP, Chen CH . Brachial-ankle vs carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens 2008; 22: 24–31.
Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A . Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension 2006; 48: 595–601.
Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C . Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing 2007; 36: 43–48.
Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004; 43: 1239–1245.
Bayliss WM . On the local reactions of the arterial wall to changes of internal pressure. J Physiol 1902; 28: 220–231.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605.
Williams B, Lacy PS . Impact of heart rate on central aortic pressures and hemodynamics: analysis from the CAFE (Conduit Artery Function Evaluation) study: CAFE-heart rate. J Am Coll Cardiol 2009; 54: 705–713.
Laurent P, Albaladejo P, Blacher J, Rudnichi A, Smulyan H, Safar ME . Heart rate and pulse pressure amplification in hypertensive subjects. Am J Hypertens 2003; 16: 363–370.
Lewis RP, Rittogers SE, Froester WF, Boudoulas H . A critical review of the systolic time intervals. Circulation 1977; 56: 146–158.
Cheng HM, Yu WC, Sung SH, Wang KL, Chuang SY, Chen CH . Usefulness of systolic time intervals in the identification of abnormal ventriculo-arterial coupling in stable heart failure patients. Eur J Heart Fail 2008; 10: 1200.
Hashimoto J, Ito S . Some mechanical aspects of arterial aging: physiological overview based on pulse wave analysis. Ther Adv Cardiovasc Dis 2009; 3: 367–378.
Westerhof BE, van den Wijngaard JP, Murgo JP, Westerhof N . Location of a reflection site is elusive: consequences for the calculation of aortic pulse wave velocity. Hypertension 2008; 52: 478–483.
Jiang XJ, O’Rourke MF, Jin WQ, Liu LS, Li CW, Tai PC et al. Quantification of glyceryl trinitrate effect through analysis of the synthesised ascending aortic pressure waveform. Heart 2002; 88: 143–148.
Bank AJ, Kaiser DR . Smooth muscle relaxation: effects on arterial compliance, distensibility, elastic modulus, and pulse wave velocity. Hypertension 1998; 32: 356–359.
Stokes GS, Barin ES, Gilfillan KL . Effects of isosorbide mononitrate and AII inhibition on pulse wave reflection in hypertension. Hypertension 2003; 41: 297–301.
Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension 2007; 49: 1248–1255.
Hayward CS, Kelly RP . Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol 1997; 30: 1863–1871.
Laurent S, Boutouyrie P . Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 2007; 49: 1202–1206.
McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006; 48: 602–608.
Acknowledgements
This work was supported by the intramural grants (V97C1-101, V98C1-028 and V99C1-091) from Taipei Veterans General Hospital, Taiwan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Human Hypertension website
Supplementary information
Rights and permissions
About this article
Cite this article
Liao, CF., Cheng, HM., Sung, SH. et al. Determinants of pressure wave reflection: characterization by the transit time-independent reflected wave amplitude. J Hum Hypertens 25, 665–671 (2011). https://doi.org/10.1038/jhh.2010.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2010.106
Keywords
This article is cited by
-
Wave reflections, arterial stiffness, heart rate variability and orthostatic hypotension
Hypertension Research (2014)