Abstract
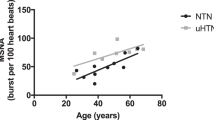
Sympathetic activation has been associated with the development and complications of hypertension. While the prevalence of hypertension and its cardiovascular risks in women are found to be less than in men and tend to become similar to men after the menopause, there have been no data on the level of sympathetic activation in postmenopausal women relative to men. Therefore, we planned to find out whether muscle sympathetic nerve hyperactivity of essential hypertension (EHT) in postmenopausal women is different from that in matched men. We quantified muscle sympathetic nerve activity (MSNA) as mean frequency of single units (s-MSNA) and multiunit bursts (b-MSNA) in 21 postmenopausal women with EHT (W-EHT) relative to 21 matched men with EHT (M-EHT), in comparison to two control groups of 21 normal women (W-NC) and 21 men (M-NC), respectively. The EHT groups had greater MSNA indices than NC groups. W-EHT had lower (P<0.05) s-MSNA (63±22.7 impulses per 100 cardiac beats) than M-EHT (78±11.2 impulses per 100 cardiac beats). W-NC had lower (P<0.05) s-MSNA (53±12.4 impulses per 100 cardiac beats) than M-NC (65±16.3 impulses per 100 cardiac beats). Similar results were obtained for b-MSNA. Postmenopausal women with EHT had lower level of central sympathetic hyperactivity than men. Similarly, normal postmenopausal women had lower MSNA than men. These findings suggest that postmenopausal women continue to have a lower sympathetic nerve activity than men even after the development of EHT, and that this could have implications for gender-specific management of hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995; 25: 305–513.
Wenger NK . Hypertension and other cardiovascular risk factors in women. Am J Hypertens 1995; 8: 94s–99s.
Wiinber N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE et al. 24-h Ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 1995; 8: 978–986.
Robitaille NM . Hypertension in women. Can J Cardiol 1996; 12 (Suppl D): 6D–8D.
Reckelhoff JF . Gender differences in the regulation of blood pressure. Hypertension 2001; 37: 1199–1208.
Khoury S, Yarows SA, O'Brien TK, Sowers JR . Ambulatory blood pressure monitoring in a nonacademic setting: effects of age and sex. Am J Hypertens 1992; 5: 616–623.
Orshal JM, Khalil RA . Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 2004; 286: R233–R249.
Welty FK . Preventing clinically evident coronary heart disease in the postmenopausal woman. Menopause 2004; 11: 484–494.
Fiebach NH, Hebert PR, Stampfer MJ, Colditz GA, Willett WC, Rosner B et al. A prospective study of high blood pressure and cardiovascular disease in women. Am J Epidemiol 1989; 130: 646–654.
Kitler ME . Differences in men and women in coronary artery disease, systemic hypertension and their treatment. Am J Cardiol 1992; 70: 1077–1080.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R . Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913.
Lloyd-Jones DM, Leip EP, Larson MG, Vasan RS, Levy D . Novel approach to examining first cardiovascular events after hypertension onset. Hypertension 2005; 45: 39–45.
Mitchell A, Philipp T . Women and hypertension. Herz 2005; 30: 401–404.
Julius S, Nesbitt S . Sympathetic overactivity in hypertension. A moving target. Am J Hypertens 1996; 9: 113s–1120s.
Grassi G . Role of the sympathetic nervous system in human hypertension. J Hypertens 1998; 16: 1979–1987.
Jennings GL . Noradrenaline spillover and microneurography measurements in patients with primary hypertension. J Hypertens 1998; 16 (Suppl): S35–S38.
Greenwood JP, Stoker JB, Mary DASG . Single unit sympathetic discharge: quantitative assessment in human hypertensive disease. Circulation 1999; 100: 1305–1310.
Mancia G, Grassi G, Giannattasio C, Seravalle G . Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34: 724–728.
Esler M . The sympathetic system and hypertension. Am J Hypertens 2000; 13: 99S–105S.
Hogarth AJ, Mackintosh AF, Mary DASG . The effect of gender on the sympathetic nerve hyperactivity of essential hypertension. J Human Hypertens 2007; 21: 239–245.
Ng AV, Callister R, Johnson DG, Seals DR . Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 1993; 21: 498–503.
Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T . Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol 1998; 275: R1600–R1604.
Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK . Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 2005; 45: 522–525.
Jones PP, Snitker S, Skinner JS, Ravussin E . Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol 1996; 270: E363–E366.
Jones PP, Spraul M, Matt KS, Seals DR, Skinner JS, Ravussin E . Gender does not influence sympathetic neural reactivity to stress in healthy humans. Am J Physiol 1996; 270: H350–H357.
Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI . Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 2001; 281: H2028–H2035.
Hogarth AJ, Mackintosh AF, Mary DASG . Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci 2007; 112: 353–361.
Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR . Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol 1999; 26: 1440–1681.
Mary DA, Stoker JB . The activity of single vasoconstrictor nerve units in hypertension. Acta Physiol Scand 2003; 177: 367–376.
Macefield VG, Wallin BG, Vallbo AB . The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol (Lond) 1994; 481: 799–809.
Lenders JW, De Boo T, Lemmens WA, Reijenga J, Willemsen JJ, Thien T . Comparison of blood pressure response to exogenous epinephrine in hypertensive men and women. Am J Cardiol 1988; 61: 1288–1291.
Sundlöf G, Wallin BG . Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol (Lond) 1978; 274: 621–637.
Minson CT, Halliwill JR, Young TM, Joyner MJ . Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 2000; 101: 862–868.
Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR et al. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol 1998; 85: 2075–2081.
Weitz G, Elam M, Born J, Fehm HL, Dodt C . Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab 2001; 86: 344–348.
Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG . Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation 2001; 103: 2903–2908.
Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA . Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation 2001; 103: 2909–2914.
Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR . Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol (Lond) 2003; 547: 309–316.
Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL et al. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension 1999; 33: 1195–1200.
Acknowledgements
We thank Mr Jeff Bannister and Mrs Julie Corrigan for technical assistance and the British Heart Foundation for sponsorship (Grant No: FS/04/085).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors state no financial disclosures or conflict of interest.
Rights and permissions
About this article
Cite this article
Hogarth, A., Burns, J., Mackintosh, A. et al. Sympathetic nerve hyperactivity of essential hypertension is lower in postmenopausal women than men. J Hum Hypertens 22, 544–549 (2008). https://doi.org/10.1038/jhh.2008.31
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2008.31
Keywords
This article is cited by
-
Inflammation and hypertension: more evidence but is there anything new?
Journal of Human Hypertension (2021)
-
The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019)
Hypertension Research (2019)
-
BP regulation VI: elevated sympathetic outflow with human aging: hypertensive or homeostatic?
European Journal of Applied Physiology (2014)
-
Hypertension in Postmenopausal Women
Current Hypertension Reports (2012)
-
Postmenopausal Hypertension
American Journal of Hypertension (2011)