Abstract
Dyslipidemias, especially hyper-low-density lipoprotein cholesterolemia and hypertriglyceridemia, are important causal risk factors for coronary artery disease. Comprehensive genotyping using the ‘next-generation sequencing’ technique has facilitated the investigation of Mendelian dyslipidemias, in addition to Mendelian randomization studies using common genetic variants associated with plasma lipids and coronary artery disease. The beneficial effects of low-density lipoprotein cholesterol-lowering therapies on coronary artery disease have been verified by many randomized controlled trials over the years, and subsequent genetic studies have supported these findings. More recently, Mendelian randomization studies have preceded randomized controlled trials. When the on-target/off-target effects of rare variants and common variants exhibit the same direction, novel drugs targeting molecules identified by investigations of rare Mendelian lipid disorders could be promising. Such a strategy could aid in the search for drug discovery seeds other than those for dyslipidemias.
Similar content being viewed by others
Introduction
Since the first description of the structure of DNA in 1953, great advances have been achieved in sequencing techniques, which have had an impact on human biology and medicine. Owing to such advances, comprehensive genotyping for exome-wide, genome-wide and targeted genomic regions has been widely used in research settings as well as in clinical settings.
Plasma lipid levels have been shown to be highly heritable as well as modifiable risk factors for coronary artery disease (CAD).1, 2, 3, 4 These findings have motivated researchers to perform genetic association studies, which could potentially define which risk factors are causal. In addition, such genetic association studies could identify pathways and therapeutic targets for dyslipidemias as well as CAD.5
In this review, we provide a current perspective on comprehensive genotyping in dyslipidemias, organizing the concepts on the basis of the allele frequencies and effect sizes of the genetic variants, and introducing the paradigm shift of innovative drug development targeting lipids to reduce CAD through genetics.
Advances in comprehensive genotyping
The structure of DNA was first described by Watson and Crick in 1953.6 Twenty-four years later, two papers introducing Gilbert and Sanger sequencing were published. This technique has become a research tool as well as a standard commercial procedure.7, 8 Recently, the so-called next-generation sequencing (NGS) technologies featuring massive parallel sequencing have been developed. NGS is widely used in a variety of applications, such as whole genome, whole exome and RNA sequencing.9 A third generation of sequencing (single-molecule sequencing) has also been developed, which avoids DNA/RNA amplification in a template library. The advantages of third-generation sequencing include avoiding polymerase chain reaction (PCR)-induced error and amplification bias with the use of less genomic DNA. Finally, a fourth-generation sequencing technology characterized by single-molecule real-time sequencing using electrical signals has been developed. Although it requires high technology, it is the simplest method of genotyping and could be the ‘ultimate technology’ for sequencing. Advances in sequencing technology are summarized in Table 1.
Clinical exome sequencing in Mendelian dyslipidemias
Whole-exome sequencing has been widely used to determine their genetic backgrounds in families with suspected monogenic inherited diseases, including dyslipidemias.10 With this approach, thousands of variants are usually detected in an individual regardless of phenotypes.11, 12 We typically exclude variants predicted to be benign or common and those with an unmatched segregation pattern within a family (Figure 1). To determine the pathogenicity of particular variants, in silico variant annotation prediction tools are quite useful.13 In addition, information on the allele frequency of certain variants from comprehensively sequenced public data can be used for this purpose.14 Phenotypic assessment, including segregation patterns, is the most important part of these procedures. It is laborious to define a segregation pattern, as much phenotypic information about the relatives, including those without the phenotype, must be collected.
Mendelian randomization studies: Causal factor or merely bystander?
To establish a causal relationship between a risk factor (usually a biomarker) and an outcome, randomized controlled trials (RCTs), which require a large amount of time and effort, are the gold standard. In contrast, the Mendelian randomization study is a technique that uses genotypes as instruments to assess a causal relationship between biomarkers and outcomes.15 In a Mendelian randomization study, a genetic variant associated with a particular biomarker is used as a proxy for the biomarker. Outcomes are compared between the group harboring the effect allele and a group with the reference allele. This approach can be considered a proxy for an RCT, in which the randomized groups have similar confounding variables (Figure 2). Accordingly, a Mendelian randomization study can be regarded as a natural RCT.
The LDL cholesterol story
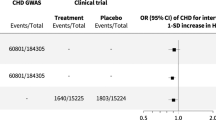
Studies with increasing sample sizes have shown that a number of polymorphisms in multiple different genes are associated with plasma low-density lipoprotein (LDL) cholesterol levels.16, 17, 18 Inheriting an allele associated with lower LDL cholesterol can be considered as equivalent to being randomly allocated to an LDL cholesterol-lowering therapy from birth, whereas inheriting the reference allele can be considered as equivalent to being randomly allocated to usual care. Comparing the rate of cardiovascular events between individuals with and without such an LDL cholesterol-lowering polymorphism should, therefore, provide an unconfounded causal estimate of the effect of long-term exposure to lower LDL cholesterol on the risk of CAD, similar to the result obtained from a long-term RCT. Mendelian randomization studies have repeatedly shown that polymorphisms that are associated with lower LDL cholesterol are also associated with a lower risk of CAD.19, 20, 21 The magnitude of the reduction of the risk of CAD is linearly correlated with the magnitude of the reduction of LDL cholesterol as a result of polymorphisms (Figure 3). This relationship between genetically mediated LDL cholesterol and CAD risk reduction seems to be similar to that observed in RCTs using statins, although the magnitude of the association is stronger in individuals with lifelong exposure from genetic variants (Figure 3).
Linear relationship between coronary artery disease (CAD) risk reduction and lowering of low-density lipoprotein (LDL) cholesterol. Blue line indicates the relationship between CAD risk reduction and genetic (lifelong) LDL cholesterol lowering. Pink line indicates the relationship between CAD risk reduction and pharmacological lowering of LDL cholesterol.
The HDL cholesterol story
Numerous epidemiological studies have shown that high-density lipoprotein (HDL) cholesterol levels are inversely associated with CAD.22, 23, 24 Such observations have motivated physicians and researchers to develop HDL cholesterol-raising therapies to reduce CAD. In 1990, Inazu et al.25 reported a family exhibiting extremely high HDL cholesterol levels caused by a loss-of-function mutation in the cholesteryl ester transfer protein (CETP) gene. Development of CETP inhibitors began after this discovery, and several large RCTs have been conducted aiming to clarify the beneficial effect of increasing HDL cholesterol with the use of these drugs in the prevention of CAD.26, 27 RCTs have also been conducted to determine if niacin, which raises HDL cholesterol, reduces the risk of CAD. The predicted risk reduction was not obtained, in contrast to the ‘LDL cholesterol story.’28 In 2010, it was shown that a common genetic variant in apolipoprotein A1 (ApoA1), which increases levels of apoA-I and HDL cholesterol, was not associated with a decreased risk of CAD.29 Kathiresan and co-workers30 reported that having an allele or alleles that raise HDL cholesterol did not reduce the risk of CAD. These studies cast doubt on the concept of HDL cholesterol as ‘good’ cholesterol and have led us to regard HDL cholesterol as merely a marker.
The triglyceride story
How about triglycerides? Triglycerides have also been shown to be associated with CAD in numerous epidemiological studies.31, 32 Establishing triglycerides as a causal factor of CAD has been difficult, as it is difficult to distinguish the effects of triglycerides on CAD from those of LDL cholesterol and HDL cholesterol by RCTs or by Mendelian randomization studies. Do et al.33 performed an interesting study creating a framework to see if triglyceride levels causally influence the risk of CAD. They constructed a model adjusting the effects of LDL cholesterol and/or HDL cholesterol levels on the risk of CAD, and found that the genetic impact of single-nucleotide polymorphisms on triglyceride levels was independently associated with the risk of CAD.33 Those facts imply that plasma triglyceride levels causally affect the risk of CAD.
Novel pharmacological targets for the treatment of dyslipidemias identified through genetics
Primary hypobetalipoproteinemia includes abetalipoproteinemia (ABL) and chylomicron retention disease, which have a recessive form of inheritance, and familial hypobetalipoproteinemia, which has a dominant form of inheritance.34 ABL is an extremely rare recessive disease caused by mutations in the microsomal triglyceride transfer protein (MTP) gene, and chylomicron retention disease is also an extremely rare recessive disease caused by mutations in SARA2 genes. The genetic backgrounds of familial hypobetalipoproteinemia have been shown to be loss-of-function mutations in the genes for apolipoprotein B (ApoB) and proprotein convertase subtilisin/kexin type 9 (PCSK9). It is of note that investigations of such rare variants in extreme cases have led to the development of novel drugs targeting the general population.
ApoB
ApoB is the primary apolipoprotein of very-low-density lipoprotein, intermediate-density lipoprotein, LDL and lipoprotein(a) particles. Mipomersen, which inhibits the synthesis of ApoB, has been developed based on those backgrounds.35 In addition to a number of clinical trials of the effect of mipomersen in patients with heterozygous familial hypercholesterolemia,36, 37 it has been shown that mipomersen is effective even in patients with homozygous familial hypercholesterolemia, where the effect of statins is limited.38 In 2013, the US Food and Drug Administration approved this drug for homozygous familial hypercholesterolemia, despite its relatively frequent adverse effect of elevated liver enzymes associated with fatty liver. From the viewpoint of genetics, it had already been shown that patients with familial hypobetalipoproteinemia caused by ApoB loss-of-function mutations have low LDL cholesterol as well as elevated liver enzymes associated with fatty liver.39, 40 Accordingly, such on-target/off-target effects had been already estimated based on observations of patients with familial hypobetalipoproteinemia and ApoB mutations.
MTP
MTP has a pivotal role in the assembly and secretion of apoB-containing lipoproteins in the liver and intestine. Lomitapide binds MTP and inhibits the synthesis of apoB-containing lipoproteins in the intestine and the liver, leading to a reduction of plasma LDL cholesterol levels.41, 42 Deleterious mutations in the MTP gene cause ABL, a rare, autosomal recessive inherited disease.43 In patients with ABL, very-low-density lipoprotein and LDL levels are significantly reduced. Mimicking this disease by inhibiting MTP has attracted a lot of attention as a novel pharmacological strategy. However, this drug seems to cause adverse effects, including diarrhea, steatorrhea, and fatty liver, as seen in patients with ABL harboring mutations in the MTP gene.
PCSK9
In 2003, PCSK9 was recognized from the discovery of causal mutations in this gene in families with severe hypercholesterolemia.44 Since its recognition, PCSK9 has been regarded as a third cause of autosomal dominant hypercholesterolemia. Loss-of-function mutations in the PCSK9 gene have been shown to be associated with lowering of LDL cholesterol and reduction of CAD risk.45, 46 These findings have facilitated the development of PCSK9 inhibitors aiming to reduce plasma LDL cholesterol and CAD.47 Considering the robust evidence from human genetics, including the fact that the subject harboring loss-of-function mutations in the PCSK9 gene is apparently healthy without liver dysfunction,48 this drug seems to be promising in terms of reducing the risk of CAD with fewer adverse effects than those from ApoB/MTP inhibitors.
Rare variants (family-based studies), common variants (population-based studies) and clinical trials
As has been repeatedly shown in the success stories of the discoveries of rare variants in extreme cases that have led to the development of novel drugs for the general population, clinical trials using drugs with consistent directions of on-target/off-target effects between rare variants and common variants are likely to succeed (Figure 4).
Schema of directions of on-target/off-target effects among rare inherited diseases, common heritable diseases and randomized controlled trials. Blue arrow indicates the on-target/off-target effects of rare inherited diseases, which are often large enough to observe in a single case (family). Red arrows indicate the on-target/off-target effects of common heritable diseases, which often require large sample sizes to determine their direction. Green arrows indicate the on-target/off-target effects observed with the use of a drug in a randomized controlled trial.
Contribution of rare variants to lipids and CAD
In addition to the simplified classification between rare variants with critical effects and common variants with small effects, many rare variants with modest effects contribute to lipids and CAD. To identify such contributions, several rare variant association studies investigating rare variants and lipids/CAD using whole-exome sequencing,49 whole-genome sequencing50 and imputation-based approach51 have been performed. These studies have found that (1) rare and low-frequency variants account for 3–4% of the variance explained in lipids, (2) rare and low-frequency variants in ApoB-related genes are associated with CAD and (3) rare and low-frequency variants in LDL receptor gene and apolipoprotein A5 gene contribute to the development of early-onset myocardial infarction. These results collectively suggest that rare and low-frequency variants with modest effects contribute to lipids and CAD, although their amounts are smaller than those of common variants.
Different ethnicity, different variants
Based on the recent growth in the worldwide population, there are several rare and private variants. Such rare variants tend to be specific to ethnicity compared with common variants. It is true that the distribution of lipid trait-related variants could be different among different ethnic groups, but the major driver of this difference could be allele frequency as reported in previous association studies using common and rare variants in different ethnic groups.52, 53 Accordingly, we believe that it is still possible to discover novel genes associated with lipids by well-designed rare variant association studies, especially in non-European populations.
Identifying lipid-related causal genes
Genome-wide association studies using common variants have identified more than 100 different loci associated with lipids till date. However, it is still challenging to detect causal genes/variants identified by genome-wide association studies located outside the coding region. In this regard, referring to a Mendelian type of disease or to a follow-up study focusing on rare coding variants seems to be a good approach.
Conclusion
Dyslipidemias, especially hyper-LDL cholesterolemia and hypertriglyceridemia, are important causal factors of CAD. Comprehensive genotyping in Mendelian dyslipidemias as well as Mendelian randomization studies using common genetic variants have contributed to our understandings of lipids and CAD. Investigation of extreme cases caused by rare genetic variants can lead to the development of novel drugs for the general population, especially when the on-target/off-target effects of rare variants and common variants exhibit the same direction. Such a strategy could aid in the search for drug discovery seeds other than those for dyslipidemias.
References
Go, A. S., Mozaffarian, D., Roger, V. L., Benjamin, E. J., Berry, J. D., Borden, W. B. et al. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127, 143–152 (2013).
Arsenault, B. J., Boekholdt, S. M. & Kastelein, J. J. Lipid parameters for measuring risk of cardiovascular disease. Nat. Rev. Cardiol. 8, 197–206 (2011).
Emerging Risk Factors Collaboration, Di Angelantonio, E., Sarwar, N., Perry, P., Kaptoge, S., Ray, K. K. et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302, 1993–2000 (2009).
Weiss, L. A., Pan, L., Abney, M. & Ober, C. The sex-specific genetic architecture of quantitative traits in humans. Nat. Genet. 38, 218–222 (2006).
Kathiresan, S. & Srivastava, D. Genetics of human cardiovascular disease. Cell 148, 1242–1257 (2012).
Watson, J. D. & Crick, F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171, 737–738 (1953).
Maxam, A. M. & Gilbert, W. A new method for sequencing DNA. Proc. Natl Acad. Sci. USA 74, 560–564 (1977).
Sanger, F., Nicklen, S. & Coulson, A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl Acad. Sci. USA 74, 5463–5467 (1977).
Dewey, F. E., Pan, S., Wheeler, M. T., Quake, S. R. & Ashley, E. A. DNA sequencing: clinical applications of new DNA sequencing technologies. Circulation 125, 931–944 (2012).
Stitziel, N. O., Peloso, G. M., Abifadel, M., Cefalu, A. B., Fouchier, S., Motazacker, M. M. et al. Exome sequencing in suspected monogenic dyslipidemias. Circ. Cardiovasc. Genet. 8, 343–350 (2015).
Tada, H., Kawashiri, M. A., Nohara, A., Saito, R., Tanaka, Y., Nomura, A. et al. Whole exome sequencing combined with integrated variant annotation prediction identifies asymptomatic Tangier disease with compound heterozygous mutations in ABCA1 gene. Atherosclerosis 240, 324–329 (2015).
Tada, H., Kawashiri, M. A., Yamagishi, M. & Hayashi, K. Whole exome sequencing in monogenic dyslipidemias. J. Atheroscler. Thromb. 22, 881–885 (2015).
Butkiewicz, M. & Bush, W. S. In silico functional annotation of genomic variation. Curr. Protoc. Hum. Genet. 88, 15.1–6.15.17 (2016).
Exome Aggregation Consortium (ExAC), Cambridge, MA. Available at: http://exac.broadinstitute.org (last accessed November 2016).
Ference, B. A. Mendelian randomization studies: using naturally randomized genetic data to fill evidence gaps. Curr. Opin. Lipidol. 26, 566–571 (2015).
Kathiresan, S., Willer, C. J., Peloso, G. M., Demissie, S., Musunuru, K., Schadt, E. E et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41, 56–65 (2009).
Teslovich, T. M., Musunuru, K., Smith, A. V., Edmondson, A. C., Stylianou, I. M., Koseki, M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 (2010).
Global Lipids Genetics Consortium, Willer, C. J., Schmidt, E. M., Sengupta, S., Peloso, G. M., Gustafsson, S. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013).
Schunkert, H., König, I. R., Kathiresan, S., Reilly, M. P., Assimes, T. L., Holm, H. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338 (2011).
Ference, B. A., Yoo, W., Alesh, I., Mirowska, K. K., Mewada, A., Kahn, J. et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J. Am. Coll. Cardiol. 60, 2631–2639 (2012).
CARDIoGRAMplusC4D Consortium, Deloukas, P., Kanoni, S., Willenborg, C., Farrall, M., Assimes, T. L. et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45, 25–33 (2013).
Genest, J. J. Jr, Martin-Munley, S. S., McNamara, J. R., Ordovas, J. M., Jenner, J., Myers, R. H. et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 85, 2025–2033 (1992).
Kannel, W. B. High density lipoproteins: epidemiologic profile and risks of coronary artery disease. Am. J. Cardiol. 52, 9B–12B (1983).
Okamura, T., Hayakawa, T., Kadowaki, T., Kita, Y., Okayama, A., Ueshima, H. et al. The inverse relationship between serum high-density lipoprotein cholesterol level and all-cause mortality in a 9.6-year follow-up study in the Japanese general population. Atherosclerosis 184, 143–150 (2006).
Inazu, A., Brown, M. L., Hesler, C. B., Agellon, L. B., Koizumi, J., Takata, K. et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 323, 1234–1238 (1990).
Nissen, S. E., Tardif, J. C., Nicholls, S. J., Revkin, J. H., Shear, C. L., Duggan, W. T. et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356, 1304–1316 (2007).
Schwartz, G. G., Olsson, A. G., Abt, M., Ballantyne, C. M., Barter, P. J., Brumm, J. et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367, 2089–2099 (2012).
AIM-HIGH Investigators, Boden, W. E., Probstfield, J. L., Anderson, T., Chaitman, B. R., Desvignes-Nickens, P. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
Haase, C. L., Tybjærg-Hansen, A., Grande, P. & Frikke-Schmidt, R. Genetically elevated apolipoprotein A-I, high-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J. Clin. Endocrinol. Metab. 95, E500–E510 (2010).
Voight, B. F., Peloso, G. M., Orho-Melander, M., Frikke-Schmidt, R., Barbalic, M., Jensen, M. K. et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380, 572–580 (2012).
Austin, M. A., Rodriguez, B. L., McKnight, B., McNeely, M. J., Edwards, K. L., Curb, J. D. et al. Low-density lipoprotein particle size, triglycerides, and high-density lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese-American men. Am. J. Cardiol. 86, 412–416 (2000).
Sharrett, A. R., Ballantyne, C. M., Coady, S. A., Heiss, G., Sorlie, P. D., Catellier, D. et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 104, 1108–1113 (2001).
Do, R., Willer, C. J., Schmidt, E. M., Sengupta, S., Gao, C., Peloso, G. M. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45, 1345–1352 (2013).
Tarugi, P., Averna, M., Di Leo, E., Cefalù, A. B., Noto, D., Magnolo, L. et al. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis 195, e19–e27 (2007).
Kastelein, J. J., Wedel, M. K., Baker, B. F., Su, J., Bradley, J. D., Rosie, Z. Y. et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation 114, 1729–1735 (2006).
Raal, F. J., Santos, R. D., Blom, D. J., Marais, A. D., Charng, M. J., Cromwell, W. C. et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375, 998–1006 (2010).
Stein, E. A., Dufour, R., Gagne, C., Gaudet, D., East, C., Donovan, J. M. et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 126, 2283–2292 (2012).
Santos, R. D., Duell, P. B., East, C., Guyton, J. R., Moriarty, P. M., Chin, W. et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur. Heart J. 36, 566–575 (2015).
Katsuda, S., Kawashiri, M. A., Inazu, A., Tada, H., Tsuchida, M., Kaneko, Y. et al. Apolipoprotein B gene mutations and fatty liver in Japanese hypobetalipoproteinemia. Clin. Chim. Acta 399, 64–68 (2009).
Kawashiri, M. A., Tada, H., Hashimoto, M., Taniyama, M., Nakano, T., Nakajima, K. et al. Extreme contrast of postprandial remnant-like particles formed in abetalipoproteinemia and homozygous familial hypobetalipoproteinemia. JIMD Rep. 22, 85–94 (2015).
Cuchel, M., Bloedon, L. T., Szapary, P. O., Kolansky, D. M., Wolfe, M. L., Sarkis, A. et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356, 148–156 (2007).
Cuchel, M., Meagher, E. A., du Toit Theron, H., Blom, D. J., Marais, A. D., Hegele, R. A. et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 381, 40–46 (2013).
Yang, X. P., Inazu, A., Yagi, K., Kajinami, K., Koizumi, J. & Mabuchi, H. Abetalipoproteinemia caused by maternal isodisomy of chromosome 4q containing an intron 9 splice acceptor mutation in the microsomal triglyceride transfer protein gene. Arterioscler. Thromb. Vasc. Biol. 19, 1950–1955 (1999).
Abifadel, M., Varret, M., Rabès, J. P., Allard, D., Ouguerram, K., Devillers, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Cohen, Pertsemlidis, A., Kotowski, I. K., Graham, R., Garcia, C. K. & Hobbs, H. H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37, 161–165 (2005).
Cohen, J. C., Boerwinkle, E., Mosley, T. H. Jr & Hobbs, H. H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Shimada, Y. J. & Cannon, C. P. PCSK9 (Proprotein convertase subtilisin/kexin type 9) inhibitors: past, present, and the future. Eur. Heart. J. 36, 2415–2424 (2015).
Zhao, Z., Tuakli-Wosornu, Y., Lagace, T. A., Kinch, L., Grishin, N. V., Horton, J. D. et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79, 514–523 (2006).
Do, R., Stitziel, N. O., Won, H. H., Jørgensen, A. B., Duga, S., Merlini, P. A. et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 518, 102–106 (2015).
Helgadottir, A., Gretarsdottir, S., Thorleifsson, G., Hjartarson, E., Sigurdsson, A., Magnusdottir, A. et al. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat. Genet. 48, 634–639 (2016).
Surakka, I., Horikoshi, M., Mägi, R., Sarin, A. P., Mahajan, A., Lagou, V. et al. The impact of low-frequency and rare variants on lipid levels. Nat. Genet. 47, 589–897 (2015).
Peloso, G. M., Auer, P. L., Bis, J. C., Voorman, A., Morrison, A. C., Stitziel, N. O. et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am. J. Hum. Genet. 94, 223–232 (2014).
Tang, C. S., Zhang, H., Cheung, C. Y., Xu, M., Ho, J. C., Zhou, W. et al. Exome-wide association analysis reveals novel coding sequence variants associated with lipid traits in Chinese. Nat. Commun. 6, 10206 (2015).
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tada, H., Kawashiri, Ma. & Yamagishi, M. Comprehensive genotyping in dyslipidemia: mendelian dyslipidemias caused by rare variants and Mendelian randomization studies using common variants. J Hum Genet 62, 453–458 (2017). https://doi.org/10.1038/jhg.2016.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2016.159
This article is cited by
-
Racial Disparities and Cardiometabolic Risk: New Horizons of Intervention and Prevention
Current Diabetes Reports (2022)
-
Personalized medicine for cardiovascular diseases
Journal of Human Genetics (2021)
-
How Genomics Is Personalizing the Management of Dyslipidemia and Cardiovascular Disease Prevention
Current Cardiology Reports (2018)
-
Lipid testing in infectious diseases: possible role in diagnosis and prognosis
Infection (2017)