Abstract
Axial spondylometaphyseal dysplasia (axial SMD) is a unique form of SMD characterized by dysplasia of axial skeleton and retinal dystrophy. Recently, C21orf2 has been identified as the first disease gene for axial SMD; however, the presence of genetic heterogeneity is known. In this study, we identified NEK1 as the second disease gene for axial SMD. By whole-exome sequencing in a patient with axial SMD, we identified compound heterozygous mutations of NEK1, c.3107C>G (p.S1036*) and c.3830A>C (p.D1277A), which co-segregated in the family. NEK1 mutations have previously been found in three types of short rib thoracic dystrophy, which have no retinal dystrophy. The skeletal phenotype of our patient was milder than those of previously reported cases with NEK1 mutations and those with axial SMD harboring C21orf2 mutations. Phenotypes associated with NEK1 mutations are variable and the phenotype–genotype corelation in skeletal ciliopathies is challenging.
Similar content being viewed by others
Main
Spondylometaphyseal dysplasia (SMD) is a group of genetic skeletal disorders that show abnormal development of spine and metaphyses of long tubular bones.1 Axial SMD (MIM 602271) is a subtype of SMD. The main clinical features of axial SMD include (1) mild postnatal growth failure, (2) severe chest deformity and (3) impaired visual acuity with retinal dystrophy.2, 3, 4 The radiological features of axial SMD include (1) cupped and flared anterior ends of ribs, (2) lacy ilia and (3) metaphyseal dysplasia of proximal femora with irregular and enchondroma-like metaphyses.5
Recently, C21orf2 was identified as the disease gene for axial SMD; however, evidence indicating for genetic heterogeneity of axial SMD was also found.5 In this study, we have identified NEK1 as the second disease gene for axial SMD.
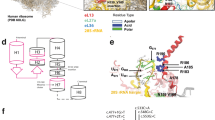
The study was approved by the Ethics Committees in RIKEN Center for Integrative Medical Sciences and Karolinska Institutet. The proband was the second child of a non-consanguineous Caucasian couple. The auxology data of the family is presented in Supplementary Table S1. He was considered to be healthy until 7 years of age, when his vision deteriorated abruptly. He was diagnosed with hypermetropia, astigmatism and neuroretinal degeneration. Electroretinogram (ERG) at age 11 years showed severe retinal dystropy (Figure 1a). The skeletal dysplasia was found at an age of 8 years. He had a slim thoracic cage and hyperflexible finger joints. He did not have short stature, facial abnormalities, cleft lip and/or palate, and dysplasia of fingers. Skeletal survey showed a narrow and long thorax, mild platyspondyly with rounded vertebral bodies, underdeveloped lower part of the pelvis, sclerotic proximal femoral metaphyses and mild metaphyseal broadening of the distal femora and proximal tibia (Figure 1b–f). Echocardiography revealed mild insufficiency of bicuspid and tricuspid valves. Laboratory tests including liver and kidney evaluation were normal. On basis of the clinical and radiological grounds mentioned above, a diagnosis of axial SMD was made.
Clinical features of the patient. (a) Electroretinogram of the patient at age 11 years. Rod response, mixed rod-cone response, single cone response and 30 Hz flicker cone response of a normal subject (left) and the patient (right). The patient showed extremely diminished mixed rod–cone response with non-recordable rod response, oscillatory potentials and cone responses, indicating a widespread retinal degeneration. (b–f) Radiographs of the patient at age 8 years. (b) Chest A-P. Narrow thorax with short ribs. (c) Lateral spine. Mild platyspondyly with rounded, dorsally wedged vertebral bodies. (d) Lower leg A-P. Mild metaphyseal broadening of the proximal tibia. (e) Hip A-P. Short ilia with narrow greater sciatic notches and horizontal acetabula, mildly flat capital femoral epiphyses, and irregular trabeculae in both femoral necks. (f) Hand A-P. Mild shortening of the 4th and 5th metacarpals.
Genomic DNA was extracted from peripheral blood of the patient and his family members (Supplementary Figure S1A). Initially, the coding exons and surrounding intronic regions of C21orf2 were examined by Sanger sequencing as described previously,5 but no candidate variant was found in C21orf2. Then whole-exome sequencing on the patient’s DNA was performed as described previously.5, 6 The summary of the sequencing performance is provided in Supplementary Table S2. By using autosomal recessive model and filtering strategy described previously,5 we detected two variants in the gene NEK1. NEK1 (NM_001199397) consists of 36 exons and encodes a protein of 1286 amino acids. Four other splicing isoforms with minor in-frame alterations (NM_012224, NM_001199398-400) are known. The detected NEK1 mutations are c.3107C>G (p.S1036*) in exon 31 and c.3830A>C (p.D1277A) in exon 35, respectively (numbered according to NM_001199397 and NP_001186326). Sanger sequencing of family members confirmed the exome-sequencing results and showed the segregation of the two variants (Supplementary Figure S1). The variants were very rare in the general population, and were predicted as disease causing by SIFT7, PolyPhen28 and MutationTaster9 (Supplementary Table S3).
NEK1 (OMIM: 604588) belongs to the NIMA (never in mitosis gene a)-related kinase family, which is conserved in evolution9 and involved in multiple cellular process, including mitosis, DNA repair, microtubule dynamics and ciliogenesis.10, 11 A recent siRNA-based functional genomics screening has revealed that NEK1 forms a functional module of ciliogenesis along with C21orf2 and SPATA7.12 C21orf2 is the known disease-causing gene of axial SMD.5
At present, 11 NEK1 mutations related to human or mouse monogenic phenotypes were reported (Table 1, Figure 2). Previous NEK1 mutations were reported in various transcripts in human and mouse. We therefore unified human NEK1 mutations and mouse Nek1 mutation in Kat2J in reference to human transcript NM_001199397 and NP_001186326 (hg19) by using NCBI BLAST and Clustal X version 2.0.13 The NEK1-related phenotypes were described according to the latest version of nosology and classification guide of genetic skeletal disorders.1 Seven NEK1 mutations are reported in short-rib thoracic dysplasia 6 (SRTD6; OMIM #263520), a skeletal disease more severe than axial SMD. SRTD6 is characterized by short ribs and limbs, median cleft lip, pre- and post-axial polysyndactyly, genital abnormalities, anomalies of epiglottis and viscera,but has no retinal dystrophy.14, 15, 16 A 3-year-old patient who is a compound heterozygote for NEK1 mutations has a phenotype overlapping with SRTD1 (OMIM %208500) and cranioectodermal dysplasia 1 (OMIM #218330 CED1), but also without retinal dystrophy.17 Two autosomal recessive mouse strains generated for polycystic kidney disease (PKD), kat and kat2J, have Nek1 mutations. Besides the PKD phenotype, skeletal changes of these mice only include decreased body size and abnormal craniofacial morphology.18
Domain structure of NEK1 protein and distribution of mutations. The amino acid sequence of NEK1 protein refers to NP_001186326. S_TKc: serine/threonine kinase catalytic domain. Grey box: coiled-coil region; black box: low-complexity region. Dot-line arrow: deleterious mutations (nonsense mutation, splicing mutation, insertion or deletion that causes frame-shift); solid-line arrow: missense mutation. Mutations identified in the present study are in bold. Splicing mutations are in bracket and are displayed on the DNA level. A large deletion described in mouse PKD model is not included in this figure since its detailed position is not available.
The phenotypic variability could be explained by the location of mutations on the NEK1 protein. Most of the previous mutations are in the N-terminal of the protein, in or near the serine/threonine kinase catalytic domain of this enzyme (Figure 2). The two mutations identified in this study were located in the C-terminal and probably have milder alteration of protein structure and function.
Wheway et al.12 proposed that disruption of SPATA7-C21orf2 interaction contributes to the retinal phenotype, while disruption of NEK1-C21orf2 interaction contributes to the skeletal phenotype. However, the effect of NEK1 mutations in the visual system has been unrecognizable because all affected patients and mouse strains suffered from perinatal death due to thoracic hypoplasia. Here we provide the first in vivo evidence that NEK1 mutation could cause a retinal phenotype in patients with mild skeletal dysplasia. The similarity of the phenotype observed in this patient with NEK1 mutations and previously described patients with C21orf2 mutations provided additional in vivo evidence for the potential interaction of NEK1 and C21orf2.
The severity of skeletal phenotypes in axial SMD is variable.5 It was reported that axial SMD patients with the same C21orf2 bi-allelic mutation showed broad phenotype variability.3, 5 Regarding the radiographic phenotype of axial SMD, the patient is at the mildest end of the phenotype spectrum. In contrast to previous axial SMD cases, which show moderate-to-severe short stature becoming manifest in early childhood,5 the patient’s height followed approximately −1 SD since birth. Our findings are consistent with known phenotype variability characteristic for skeletal ciliopathies.19, 20
References
Bonafe, L., Cormier-Daire, V., Hall, C., Lachman, R., Mortier, G., Mundlos, S. et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am. J. Med. Genet. A 167A, 2869–2892 (2015).
Ehara, S., Kim, OH., Maisawa, S., Takasago, Y. & Nishimura, G. Axial spondylometaphyseal dysplasia. Eur. J. Pediatr. 156, 627–630 (1997).
Isidor, B., Baron, S., Khau van Kien, P., Bertrand, A. M., David, A. et al. Axial spondylometaphyseal dysplasia: confirmation and further delineation of a new SMD with retinal dystrophy. Am. J. Med. Genet. A 152A, 1550–1554 (2010).
Suzuki, S., Kim, O. H., Makita, Y., Saito, T., Lim, G. Y., Cho, T. J. et al. Axial spondylometaphyseal dysplasia: additional reports. Am. J. Med. Genet. A 155A, 2521–2528 (2011).
Wang, Z., Iida, A., Miyake, N., Nishiguchi, KM., Fujita, K., Nakazawa, T. et al. Axial spondylometaphyseal dysplasia is caused by C21orf2 mutations. PLoS ONE 11, e0150555 (2016).
Guo, L., Girisha, K. M., Iida, A., Hebbar, M., Shukla, A., Shah, H. et al. Identification of a novel LRRK1 mutation in a family with osteosclerotic metaphyseal dysplasia. J. Hum. Genet. (e-pub ahead of print 10 November 2016; doi:10.1038/jhg.2016.136).
Kumar, P., Henikoff, S. & Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P. et al A method and server for predicting damaging missense mutations. Nat. Methods. 7, 248–249 (2010).
Schwarz, J. M., Cooper, D. N., Schuelke, M. & Seelow, D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods 11, 361–362 (2014).
Fry, A. M., O’Regan, L., Sabir, S. R. & Bayliss, R. Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 125, 4423–4433 (2012).
Kim, G., Kim, J. Y. & Choi, H. S. Peptidyl–prolyl cis/trans isomerase NIMA-Interacting 1 as a therapeutic target in hepatocellular carcinoma. Biol. Pharm. Bull. 38, 975–979 (2015).
Wheway, G., Schmidts, M., Mans, D. A., Szymanska, K., Nguyen, T. M., Racher, H. et al. An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat. Cell Biol. 17, 1074–1087 (2015).
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H. et al. Clustal W and Clustal X version 2.0. Bioinformatics. 23, 2947–2948 (2007).
Thiel, C., Kessler, K., Giessl, A., Dimmler, A., Shalev, S. A., Beinder, E. et al. NEK1 mutations cause short-rib polydactyly syndrome type Majewski. Am. J. Hum. Genet. 88, 106–114 (2011).
Chen, C. P., Chang, T. Y., Chen, C. Y., Wang, T. Y., Tsai, F. J., Wu, P. C. et al. Short rib-polydactyly syndrome type II (Majewski): prenatal diagnosis, perinatal imaging findings and molecular analysis of the NEK1 gene. Taiwan J. Obstet. Gynecol 51, 100–105 (2012).
El Hokayem, J., Huber, C., Couvé, A., Aziza, J., Baujat, G., Bouvier, R. et al. NEK1 and DYNC2H1 are both involved in short rib polydactyly Majewski type but not in Beemer Langer cases. J. Med. Genet. 49, 227–233 (2012).
McInerney-Leo, A. M., Harris, J. E., Leo, P. J., Marshall, M. S., Gardiner, B., Kinning, E. et al. Whole exome sequencing is an efficient, sensitive and specific method for determining the genetic cause of short-rib thoracic dystrophies. Clin. Genet. 88, 550–557 (2015).
Vogler, C., Homan, S., Pung, A., Thorpe, C., Barker, J., Birkenmeier, E. H. et al. Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J. Am. Soc. Nephrol. 10, 2534–2539 (1999).
Huber, C. & Cormier-Daire, V. Ciliary disorder of the skeleton. Am. J. Med. Genet. C 160C, 165–174 (2012).
Geister, K. A. & Camper, S. A. Advances in skeletal dysplasia genetics. Annu. Rev. Genomics Hum. Genet 16, 199–227 (2015).
Chen, C. P., Chern, S. R., Chang, T. Y., Su, Y. N., Chen, Y. Y., Su, J. W. et al. Prenatal diagnosis and molecular genetic analysis of short rib-polydactyly syndrome type III (Verma-Naumoff) in a second-trimester fetus with a homozygous splice site mutation in intron 4 in the NEK1 gene. Taiwan J. Obstet. Gynecol. 51, 266–270 (2012).
Upadhya, P., Birkenmeier, E. H., Birkenmeier, C. S. & Barker, J. E. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc. Natl. Acad. Sci. U S A. 97, 217–221 (2000).
Acknowledgements
We thank the family for participating in the study. This study is supported by KAKENHI Grant-in-Aid for Scientific Research (B) (NMi, No 25293235), Takeda Science Foundation (ZW), and research grants from Japan Agency For Medical Research and Development (AMED) (SI, NMa, No 16ek0109068h0003). This study is also supported by the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet and by grants from Kronprinsessan Lovisas, Stiftelsen Frimurare Barnhuset in Stockholm, Hjärnfonden, Axel Tielmans Minnesfond, Samariten and Promobilia Foundations (GG, AN).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Wang, Z., Horemuzova, E., Iida, A. et al. Axial spondylometaphyseal dysplasia is also caused by NEK1 mutations. J Hum Genet 62, 503–506 (2017). https://doi.org/10.1038/jhg.2016.157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2016.157
This article is cited by
-
Recent Updates on the Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
Molecular Neurobiology (2022)
-
Skeletal ciliopathies: a pattern recognition approach
Japanese Journal of Radiology (2020)
-
Dysosteosclerosis is also caused by TNFRSF11A mutation
Journal of Human Genetics (2018)
-
Further expansion of the mutational spectrum of spondylo-meta-epiphyseal dysplasia with abnormal calcification
Journal of Human Genetics (2018)
-
Novel KIAA0753 mutations extend the phenotype of skeletal ciliopathies
Scientific Reports (2017)