Abstract
Chondroitin sulfate proteoglycans (CSPGs) in the peripheral nervous system likely participate as regulatory molecules in the process of axonal degeneration and regeneration. We investigated the chondroitin beta1,4-N-acetylgalactosaminyltransferase-1 (ChGn-1) gene in 114 patients affected with neuropathies including Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, hereditary motor and sensory neuropathy (HMSN) and unknown etiology. The controls were 196 patients with other neurological diseases. We found novel missense mutations in two patients with neuropathy (Bell's palsy, unknown HMSN) in exons 5 (H234R) and 10 (M509R), respectively. None of the patients with other neurological diseases had either of these mutations. We then synthesized the two soluble forms of ChGn-1, containing each of the above mutations. Each of the soluble mutants was expressed in COS-1 cells and the mutant proteins were purified. The purified mutant proteins were used for western blotting analysis using an anti-ChGn-1 antibody and evaluated for glycosyltransferase activities. Although the expression of the ChGn-1 mutant proteins was confirmed by western blotting, they exhibited no N-acetylgalactosamineT-II activities. It is possible that these mutations are associated with the pathogenetic mechanisms of the peripheral neuropathies.
Similar content being viewed by others
Introduction
Peripheral neuropathies are often caused by genetic factors. In particular, hereditary motor and sensory neuropathy (HMSN) or Charcot-Marie-Tooth disease is known to be associated with a number of causative genes.1, 2 HMSN is a heterogeneous group of degenerative peripheral nerve disorders, which altogether constitute the most common inherited neurological disease, with an incidence of 1 in 2500.3
Glycoconjugates, such as glycoproteins, proteoglycans and gangliosides, are important constituents of both the central and peripheral nervous systems. However, no association between human peripheral neuropathies and glycosyltransferases, which are involved in the synthesis of carbohydrate chains of glycoproteins, proteoglycans, gangliosides and so on, has been reported to date. Recently, it has been shown that beta1,4-N-acetylgalactosaminyltransferase (also called GM2/GD2 synthetase)-deficient mice are affected by sensory-dominant neuropathies.4
Chondroitin sulfate proteoglycans (CSPGs) have been shown to be present in the matrix of the nervous system. Several species of molecules are known such as neurocan, versican, phosphacan and so on. They show developmental and post-traumatic changes both spatially and temporally. CSPGs are known to act as growth inhibitory molecules.5 On the other hand, some of the CSPGs promote neurite outgrowth.6 CSPGs are likely to act not only as a chemical barrier but also as regulatory molecules for nerve regeneration.7 Chondroitin beta1,4-N-acetylgalactosaminyltransferase-1 (ChGn-1) is involved in an important step for the synthesis of CSPGs.8 Chondroitin sulfate chains consist of repeating disaccharide units of N-acetylgalactosamine (GalNAc) and glucuronic acid, which are sulfated at either the C6 or C4 position of GalNAc. The integrity of the chondroitin sulfate chain is maintained by elongation (biosynthesis) of the chain, which is catalyzed by ChGn-1, ChGn-2 and sulfotransferases.9 Recently, it has been reported that a broad spectrum of skeletal dysplasias result from mutations causing undersulfation of chondroitin sulfate chains in humans and in mice.10, 11, 12, 13, 14 In contrast, no association between CSPGs and human peripheral neuropathies has been reported to date.
In this study, we found novel missense mutations that resulted in a profound decrease of enzymatic activities in two patients with neuropathies.
Materials and methods
Subjects and patient populations
We recruited 310 patients with neurological disorders. We investigated 114 patients with neuropathy (40 with Guillain-Barré syndrome, 40 with chronic inflammatory demyelinating polyneuropathy, 5 with hereditary motor sensory neuropathy and 29 with unknown-etiology) and 196 disease control subjects. This study was approved by the internal review board of Kinki University School of Medicine. All patients provided written informed consent before participation in the study. Genomic DNA extraction and genotyping were performed using standard protocols.
Sequence analysis
Genomic DNA was extracted from whole blood using the QiaAmp Mini DNA kit (Qiagen, Tokyo, Japan). PCR amplicons generated with oligonucleotide primers were digested from ChGn-1 gene on the basis of GenBank sequence. We sequenced all exons and their boundaries, using the ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Materials
UDP-[3H]GalNAc (10 Ci mmol−1) and unlabeled UDP-GalNAc were purchased from NEN Life Science Products (Waltham, MA, USA) and Sigma (Tokyo, Japan), respectively. Chondroitin was purchased from Seikagaku (Tokyo, Japan).
Construction of a Soluble Form of ChGn-1
The complementary DNA fragment of a truncated form of ChGn-1, lacking the first 41 N-terminal amino acids, was amplified by reverse transcription-PCR with total RNA derived from G361 human melanoma cells (ATCC CRL-1424) as a template using a 5′-primer (5′-GCTCTAGACAGCTGGCACTGCCCAGG-3′) containing an in-frame XbaI site and a 3′-primer (5′-CGGGATCCCATCTCTGACCCATCAGTCC-3′) containing a BamHI site located 58 bp downstream of the stop codon.
Site-directed Mutagenesis
A two-stage PCR mutagenesis method was used to construct the ChGn-1 H234R or ChGn-1 M509R mutants. Two separate PCR reactions were performed to generate two overlapping gene fragments using the soluble form of ChGn-1 complementary DNA as a template. In the first PCR, the sense 5′-primer described above and either of the antisense internal mutagenic primers listed below were used: H234R 5′-GTTTGAATTCGCGTTTGTGGTCCCC-3′ or M509R 5′-CCTGAACACCAGCCTGCCCAGCTGGCCG-3′ (the mutated nucleotides are underlined). In the second round of PCR, the respective sense internal mutagenic primers (complementary to the antisense internal mutagenic primer) and the antisense 3′-primer described above were used.
Expression of soluble forms of the ChGn-1 and ChGn-1 mutants and enzyme assays
The expression plasmids (6.0 μg each) were transfected into COS-1 cells on 100-mm plates using FuGENE 6 (Roche Molecular Biochemicals, Tokyo, Japan) according to the manufacturer's instructions. At 2 days after transfection, 1 ml of the culture medium was collected and incubated with 10 μl of HIS-Select Cobalt Affinity beads (Sigma) for 1 h at 4 °C. The beads recovered by centrifugation were washed and then resuspended in the assay buffer described below, and GalNAcT transferase activity was assessed using polymer chondroitin (167 μg) as an acceptor. Reaction mixtures were incubated at 37 °C for 1 h, and radiolabeled products were then separated from UDP-[3H]GalNAc by gel filtration using a syringe column, as described previously.9
Western blot analysis
After 2 days of culture, the culture medium was collected and incubated with 10 μl of HIS-Select Cobalt Affinity beads (Sigma) for 1 h at 4 °C. The beads recovered by centrifugation were washed with phosphate-buffered saline, resolved on 7.5% SDS-polyacrylamide gels, and proteins were transferred to a polyvinylidene difluoride membrane. The membrane was incubated for 1 h with an anti-ChGn-1 mouse antibody (Transgenic, Kobe, Japan). The antibody was diluted 1:1000 with 25 mM Tris-buffered saline. The bound antibody was detected with anti-mouse IgG conjugated with horseradish peroxidase and enhanced chemiluminescence.
Results
Mutation analysis of patient DNA
In mutation analysis of the ChGn-1 gene, we found two novel heterozygous missense mutations in patients with neuropathies, H234R (1355 A >G) in exon 5 from one patient and M509R (2180 T >G) in exon 10 from the other. Those mutations were not observed in 196 unrelated disease control DNA samples.
The patient with the H234R missense mutation was a 38-year-old man. He had been affected by acquired idiopathic generalized anhidrosis since his childhood. He had no apparent family history. He was also affected by hemi-facial palsy since the age of 34. The hemi-facial palsy developed similar to an episode of acute idiopathic facial palsy, so called Bell's palsy, but was irreversible.
The patient with the M509R mutation was a 25-year-old man. He had motor and sensory neuropathy without any apparent family history. He had experienced intermittent postural tremor since he was in elementary school. Nerve conduction studies revealed an absence of sensory nerve action potentials in the median, ulnar and sural nerves. The compound muscle action potentials were reduced (left median nerve: 2.88 mV (control: >4 mV), left ulnar nerve: 0.85 mV (>3 mV) and left tibial nerve: 0.19 mV (>7 mV)). Motor nerve conduction velocities were also decreased (left median nerve: 14.7 m s−1 (>45 m s−1), left ulnar nerve: 13.6 m s−1 (>45 m/s) and left tibial nerve: 9.34 m s−1 (>40 m s−1)). He had been diagnosed with HMSN of unknown type.
Sequence analysis on PMP22 and MPZ, and PMP22 duplication and deletion study using fluorescence in situ hybridization method showed no abnormal findings in either patient.
Expression and glycosyltransferase activities of soluble forms of the ChGn-1 H234R and M509R mutants
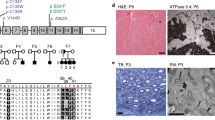
To clarify whether these mutations of ChGn-1 influence glycosyltransferase activities, we constructed soluble forms of the two ChGn-1 H234R and M509R mutants. To examine the expression and activity of the mutant proteins, each of the soluble mutants was expressed in COS-1 cells and the culture medium was purified with HIS-Select Cobalt Affinity beads. The purified mutant proteins were used for western blotting analysis, and glycosyltransferase activities were assessed using chondroitin as an acceptor. When the soluble form of the ChGn-1 H234R and M509R mutants were expressed in COS-1 cells, proteins, ∼70 kDa in size were secreted as shown by western blotting using an anti-ChGn-1 antibody (Figure 1). Although the ChGn-1 H234R and M509R mutant proteins were expressed (Figure 1, lanes 2 and 3), these mutant proteins showed no GalNAcT-II activities (Table 1).
Western blot analysis of ChGn-1 H234R and ChGn-1 M509R. A soluble form of ChGn-1, ChGn-1 H234R or ChGn-1 M509R was expressed as a fusion protein tagged with 6 × His in COS-1 cells as described in ‘Materials and methods.’ The recombinant proteins secreted in the medium were purified and then separated by SDS-PAGE, and the expression of each His-tagged protein was examined using an anti-ChGn-1 antibody. Lane 1, ChGn-1-His; lane 2, ChGn-1 M509R-His; lane 3, ChGn-1-H234R-His.
We analyzed amino acid sequence alignment of ChGn-1 gene. The novel coding mutations identified in this study changed the charge of the amino acid sequence. And the both mutation sites of ChGn-1 genes are highly conserved across species from zebrafish to humans (Figure 2). The mutation of H234R and M509R would be possible to form a new salt-bridge and contribute to the formation of the protein turn around the positive-charged residue. Moreover, these two mutations were not represented in the single-nucleotide polymorphism database (http://www.ncbi.nlm.nih.gov/projects/SNP/).
Amino acid sequence alignment of ChGn-1 gene. H234R and M509R positions are highly conserved across species. Sequence include human (homo sapience; NP_060841.3), chimpanzee (Pan troglodytes; XP_519635.2), dog (Canis lupus; XP_539946.2), mouse (Mus musculus; NP_766341.3), rat (Rattus norvegicus; XP_224757.4), chicken (Gallus gallus; XP_420453.2) and zebra fish (Danio rerio; XP_001333479.2). Sequence were aligned using the NCBI homologene web site (http://www.ncbi.nlm.nih.gov/homologene).
Discussion
In this study, we found two novel mutations in ChGn-1 gene, both of which were associated with a profound decrease of their enzyme activity, in two patients with neuropathies of unknown etiology.
Proteoglycans are considered to be involved in the development of the nervous system. Defects in the production of CSPGs therefore may be associated with developmental errors in both the central and peripheral nervous systems. Complete loss of chondroitin polymerization has been studied in nematodes, indicating that condroitin is required for embryonic cytokinesis and cell division.15, 16
In addition, CSPGs are known to have an important role in nerve injury. CSPG is known to be a major component of glial scarring. It is considered to be a major obstacle for recovery of the adult nervous system after injury, especially in light of its well-known activity in limiting axonal growth. In vitro, many CSPG family members have been reported to inhibit neurite outgrowth. However, the inhibitory activity of CSPGs does not necessarily mean that they are simple barrier molecules that contribute to nerve regeneration. They may avoid excessive fiber regeneration and inappropriate reinnervation.17 In addition, in some situations, CSPG has been shown to strongly promote neurite outgrowth.18 Thus, CSPG has a pivotal role in the repair of the injured spinal cord and in the recovery of motor function during the acute phase after injury.17
The peripheral nervous system is not as completely protected against injuries as the central nervous system. It should therefore be more vulnerable to minor trauma, such as compressions or bruises. Defect in the glycosyltransferases of the CSPGs, such as ChGn-1, may be associated with the pathogenesis of the peripheral neuropathies by disturbing the recovery from the minor trauma.
As the mutations we identified in this study are heterozygous mutations, the amount of CSPGs may not decrease profoundly in either case. We therefore consider that those mutations may not usually cause any obvious clinical disturbances. However, these mutations may reduce the effectiveness of the reparation of the peripheral nervous system because of poor productivity of CSPGs at the time of the emergency. It may be associated with the development of the irreversible hemi-facial palsy in the patient with the H234R mutation, whereas Bell’s palsy is reversible in most of the cases. Another possible mechanism is toxic or dominant-negative effect. For example, as for superoxide dismutase mutation for familial amyotrophic lateral sclerosis, the pathogenetic mechanism is not from the loss of enzymatic activities.19 This possibility should be investigated in future.
An association between the abnormality in the CSPG genes and skeletal diseases has been reported.12 However, to the best of our knowledge, the association with neurological disorders has never been reported. The present investigation did not prove that the heterozygous ChGn-1 mutations cause neuropathy in two patients. However, the possible association between the mutations and pathogenesis of neuropathy may exist. Studies on larger number of patients and complete family studies are necessary.
References
Barisic, N., Claeys, K. G., Sirotkoviæ-Skerlev, M., Löfgren, A., Nelis, E., De Jonghe, P. et al. Charcot-Marie-Tooth disease: a clinico-genetic confrontation. Ann. Hum. Genet. 72, 416–441 (2008).
Nave, K. A., Sereda, M. W. & Ehrenreich, H. Mechanisms of disease: inherited demyelinating neuropathies--from basic to clinical research. Nat. Clin. Pract. Neurol. 3, 453–464 (2007).
Skre, H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin. Genet. 6, 98–118 (1974).
Sugiura, Y., Furukawa, K., Tajima, O., Mii, S., Honda, T. & Furukawa, K. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience 135, 1167–1178 (2005).
Kuffler, D. P., Sosa, I. J. & Reyes, O. Schwann cell chondroitin sulfate proteoglycan inhibits dorsal root ganglion neuron neurite outgrowth and substrate specificity via a soma and not a growth cone mechanism. J. Neurosci. Res. 87, 2863–2871 (2009).
Cafferty, W. B., Yang, S. H., Duffy, P. J., Li, S. & Strittmatter, S. M. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J. Neurosci. 27, 2176–2185 (2007).
Rolls, A., Shechter, R., London, A., Segev, Y., Jacob-Hirsch, J., Amariglio, N. et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 58, e171 (2008).
Kitagawa, H., Tsutsumi, K., Ujikawa, M., Goto, F., Tamura, J., Neumann, K. W. et al. Regulation of chondroitin sulfate biosynthesis by specific sulfation: acceptor specificity of serum beta-GalNAc transferase revealed by structurally defined oligosaccharides. Glycobiology. 7, 531–537 (1997).
Uyama, T., Kitagawa, H., Tamura, J. & Sugahara, K. Molecular cloning and expression of human chondroitin N-acetylgalactosaminyltransferase: the key enzyme for chain initiation and elongation of chondroitin/dermatan sulfate on the protein linkage region tetrasaccharide shared by heparin/heparan sulfate. J. Biol. Chem. 277, 8841–8846 (2002).
Hastbacka, J., Superti-Furga, A., Wilcox, W. R., Rimoin, D. L., Cohn, D. H. & Lander, E. S. Atelosteogenesis type II is caused by mutations in the diastrophicdysplasia sulfate transporter gene (DTDST): evidence for a phenotypic series involving three chondrodysplasias. Am. J. Hum. Genet. 58, 255–262 (1996).
Superti-Furga, A., Hästbacka, J., Wilcox, W. R., Cohn, D. H., van der Harten, H. J., Rossi, A. et al. Achondrogenesis type IB is caused by mutations in the diastrophic dysplasia sulphate transporter gene. Nat. Genet. 12, 100–102 (1996).
Thiele, H., Sakano, M., Kitagawa, H., Sugahara, K., Rajab, A., Höhne, W. et al. Loss of chondroitin 6-O-sulfotransferase-1 function results in severe human chondrodysplasia with progressive spinal involvement. Proc. Natl Acad. Sci. USA 101, 10155–10160 (2004).
Kluppel, M., Wight, T. N., Chan, C., Hinek, A. & Wrana, J. L. Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development 132, 3989–4003 (2005).
Hiraoka, S., Furuichi, T., Nishimura, G., Shibata, S., Yanagishita, M., Rimoin, D. L. et al. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat. Med. 13, 1363–1367 (2007).
Mizuguchi, S., Uyama, T., Kitagawa, H., Nomura, K. H., Dejima, K., Gengyo-Ando, K. et al. Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature 423, 443–448 (2003).
Hwang, H. Y., Olson, S. K., Esko, J. D. & Horvitz, H. R. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature 423, 439–443 (2003).
English, A. W. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J. Comp. Neurol. 490, 427–441 (2005).
Mikami, T., Yasunaga, D. & Kitagawa, H. Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J. Biol. Chem. 284, 4494–4499 (2009).
Barber, S. C., Mead, R. J. & Shaw, P. J. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta. 1762, 1051–1067 (2006).
Acknowledgements
We gratefully thank Dr Ken-ichi Kaida for providing us with the information on clinical findings of a patient. Funding: This word was supported in part by the Ministry of Health, Labor and Welfare of Japan (Health Sciences Research Grant on Psychiatric and Neurological Diseases and Mental Health, H21-012) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Scientific Research, 21390273).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saigoh, K., Izumikawa, T., Koike, T. et al. Chondroitin beta-1,4-N-acetylgalactosaminyltransferase-1 missense mutations are associated with neuropathies. J Hum Genet 56, 143–146 (2011). https://doi.org/10.1038/jhg.2010.148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2010.148
Keywords
This article is cited by
-
Chondroitin Sulfate Is Required for Onset and Offset of Critical Period Plasticity in Visual Cortex
Scientific Reports (2017)
-
Biosynthesis of glycosaminoglycans: associated disorders and biochemical tests
Journal of Inherited Metabolic Disease (2016)
-
Acute 4-nonylphenol toxicity changes the genomic expression profile of marine medaka fish, Oryzias javanicus
Molecular & Cellular Toxicology (2014)