Abstract
MAP4K5 (mitogen-activated protein kinase kinase kinase kinase 5), an early component of MAP kinase signal cascades was shown to activate Jun kinase in mammalian cells. The association between SNPs of MAP4K5 and type 2 diabetes (T2DM) was investigated due to the known relationship of the JNK pathway with T2DM. A total of 1,399 cases were included in the study. Oral glucose tolerance test (OGTT) and insulin release test (IRT) were performed, and blood DNA samples were extracted and genotyped on the MAP4K5 -822G/A site. These cases were subdivided into central-obesity and nonobesity groups, based upon their individual waist circumference. Allele-specific real-time PCR was employed for genotyping. No difference was found between the two groups in the distribution of three genotypes on MAP4K5 -822G/A. In the central-obesity group, fewer diabetic patients (38.9%) were present in the AA genotype group than the GG/GA group (58.5%, P=0.024). Glucose levels after 30 and 60 min of 75 g glucose tolerance, area under the curve for glucose, and insulin secretion indexes were lower (P<0.05) in AA than those in GG/GA genotype group in the central-obesity cases. Other variables did not show significant differences between the two groups. In the Han population from Shanghai, the AA genotype of MAP4K5 -822G/A in central-obesity cases appears less likely to develop diabetes compared with the other genotypes. Therefore, the G allele may be a factor that does not protect central-obesity cases from developing into diabetes.
Similar content being viewed by others
Introduction
Protein serine/threonine kinases of the mitogen-activated protein kinase (MAPK) family are critical to the coordinated responses of cells to mitogenesis, developmental changes, and stress stimuli. The MAPK pathway can also be activated by insulin, oxidative stress, and cytokines. Increased basal MAPK activity appears to contribute to the development of insulin resistance. MAP4K5 (mitogen-activated protein kinase kinase kinase kinase 5), located in the upstream of the MAP kinase signal cascade, activates the JNK group of MAPK in mammalian cells, which are also members of the MAPK family. Considering the relationship of these factors and their signaling cascades to diabetes, there has been increasing interest in the JNK pathway as an important therapeutic target for the treatment of this disease (Jaeschke et al. 2005; Manning and Davis 2003; Kaneto et al. 2004). However, as a major mediator for insulin resistance, tumor necrosis factor-α (TNF-α) can induce MAP4K5 to facilitate an interaction in vivo with TRAF2 and hence transduce downstream signals (Shi and Kehrl 2003).
SNPs for MAPK genes, at least in the JNK pathway, have not been reported to possess a relationship with diabetes. However, considering the important relationship existing among the MAPK pathway, TNF-α, and diabetes, the first protein of this pathway involving MAP4K5 was selected in this study to evaluate the association of -882G/A polymorphism in the promoter region with central obesity and type 2 diabetes (T2DM) in a Chinese Han population from Shanghai.
Subjects and methods
Subjects
All subjects were of Han origin and had not previously been diagnosed positive for diabetes. Each was examined using a 75-g oral glucose tolerance test (OGTT) and insulin release test (IRT) the morning after an overnight (10 h) fast. Type 1 diabetes was identified by the determination of anti-glutamic acid decarboxylase antibodies. Samples for glucose and insulin measurements were drawn, before and after glucose ingestion, into tubes containing EDTA at time zero and every subsequent 30 min for 3 h. For this study, subjects were classified as having normal glucose tolerance (NGT), or type 2 diabetes (T2DM) on the basis of the American Diabetes Association (ADA) criteria for T2DM (American Diabetes Association 2005). Central obesity was diagnosed based on waist circumference, male≥90 cm, female≥80 cm, as suggested by 2005 worldwide definition of the metabolic syndrome in Chinese populations (IDF 2005).
Other assays
Plasma glucose was measured using the hexokinase method and plasma insulin concentrations were measured by radioimmunoassay (USA DSL-1600).
Calculations and statistical analysis
Body mass index (BMI) was calculated as weight/height2. The trapezoidal rule was used to calculate the incremental area under the curve for glucose (AUCG) for the duration of the OGTT. The homeostasis model assessment of insulin resistance (HOMA IR) was calculated from fasting glucose and insulin levels as described by Matthews et al. (1985), using the equation: resistance=[glucose]×[insulin]/22.5. B-cell function was estimated during OGTT using the Stumvoll first-phase insulin secretion index (Stumvoll et al. 2000):
and second-phase index:
The insulin sensitivity was estimated using the Cederholm index (Cederholm and Wibell 1990), which uses OGTT data:
Genotyping
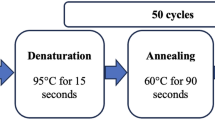
Genomic DNA was extracted from peripheral blood according to standard procedures. The -882G/A polymorphism in the MAP4K5 gene was determined by allele-specific real-time PCR (Chen et al. 2004), using ABI 7000 (Applied Biosystems, Foster City, CA) and SYBR Green mix (Golden Taq polymerase, Roche).
Statistical analysis
All data were analyzed using the SPSS/Win software package (version 10.0, SPSS, Chicago, IL, USA). Results are given as means±SD or as percentages. Variables, which were not normally distributed, were logarithmically transformed before statistical analyses. ANOVA was used to compare groups, and a Student’s t-test was used for independent samples and a χ2 test was used for comparing the frequencies of diabetes between different genotype groups. The nonparametric Mann-Whitney U and Kruskal-Wallis H tests were applied to compare changes in glucose levels and insulin secretion indexes. The clinical features of the cases studied are shown in Table 1.
Results
When all the subjects were grouped by waist circumference, different degrees of insulin resistance could be clearly seen between the two groups: the central-obesity group had a higher HOMA IR than the control (0.6±1.25 vs. 0.31±0.25, P<0.0001), and a lower insulin sensitivity index (27.03±10.26 vs. 30.8±12.05, P<0.0001); the central-obesity group seemed to correlate with a lower insulin secretion function and a greater degree of insulin resistance. No significant difference in the proportions of NGT and T2DM between the different obesity groups was observed (P=0.171).
Genotyping was performed on 1,399 out of a total of 1,595 cases. The proportion of individuals carrying the three possible genotypes (-882G/G, -882 G/A, and -882 A/A) was not significantly different for controls compared with central obesity, or controls compared with T2DM. However, in the central-obesity group, a difference in the genotype was observed between the NGT and T2DM subgroups: AA versus GA, P=0.008; AA versus GG, P=0.05; AA versus GA/GG, P=0.024 (shown in Table 2). There were fewer T2DM cases (38.9%) among homozygous subjects for -882 AA in the central-obesity group compared with those carrying the -882 G allele (58.5%, P=0.024), but this trend was not observed in the nonobesity group: in total 57.1% of AA genotype and 53.4% of GG/GA genotype in this group were T2DM (P>0.05) (see Table 2). Overall, in the central-obesity group, a significant association of the G allele with a higher risk of T2DM was observed when compared with the AA genotypes, OR: 2.217 (95% confidence index: 1.112–4.420). In the nonobesity group this difference was not significant.
Genotype distributions for MAP4K5 genotypes were in Hardy-Weinberg equilibrium (P=0.456), and had the following frequencies: 43.7% in the G allele, 56.3% in NGT and T2DM, 44.6% in the A allele, 55.4% in NGT and T2DM.
No differences were observed across the AA and AA/AG groups as far as age, gender, BMI, and blood pressure were concerned. However, glucose levels (Glu; age- and gender-corrected) at 30 and 60 min and AUCG were observed to be lower in the AA group than those with a G allele in the larger-waist-circumference group, and significant differences were observed: Glu 0 min (6.0±1.31 vs. 6.8±2.40, P=0.05), Glu 30 min (9.6±2.33 vs. 10.9±3.25, P=0.019), Glu 60 min (12.3±4.68 vs. 10.4±3.23, P=0.018) and AUCG (1,569.2±494.18 vs. 1,831.2±753.01, P=0.047) (see Table 3). We also observed a higher insulin first-phase (AA: 2,171.5±1,350.15 vs. GA/GG: 1,774.9±1,288.92, χ2=5.57, P=0.019) and second-phase secretion index (AA: 309.5±306.74 vs. GA/GG: 224.0±286.95, χ2=5.53, P=0.018). Regarding the insulin resistance and sensitivity indexes, we did not see significant differences between the two groups across the two different waist-circumference populations.
Discussion
In many cell surface signal transduction studies, MAP4K5 is recognized as an important factor that transduces the extracellular signal from the TNF-α receptor to activate JNK and NF-κB pathways (Shi et al. 2000; Shi and Kehrl 2003). TNF-α has long been regarded as a multifunctional cytokine constitutively produced by adipose tissue that may mediate insulin resistance (le Roith and Zick 2001). In other studies, TNF-α was also shown to be involved with major pro-inflammatory cytokines, implicated in islet β-cell autoimmune destruction (Christen et al. 2001; Suk et al. 2001). The -238 and -308 G→A polymorphisms of the TNF-α gene promoter have been identified. These variants have been linked, in different ethnic groups, to estimates of insulin resistance, obesity and insulin-dependent diabetes, though the results were not all consistent (Li et al. 2003; Shiau et al. 2003; Heijmans et al. 2002; Dalziel et al. 2002; Koch et al. 2000; Rasmussen et al. 2000). Recently in a Finnish diabetes-prevention study -308 G→A polymorphisms in TNF-α were shown to be a predictor for the conversion from overweight impaired glucose tolerance (IGT) to type 2 diabetes (Kubaszek et al. 2003).
The JNK/SAPK pathway activity induced by TNF-α could result in the production of reactive oxidative species (ROS) and loss of mitochondrial transmembrane potential (ψm) in the MIN6 cell line (Kim et al. 2006). Mice fed on a high-fat diet, and ob/ob mice, genetically prone to obesity, exhibit spontaneously high JNK1 activity in the liver, skeletal muscle and fat, while Jnk1−/− high-fat and ob/ob mice are protected from the development of dietary obesity-induced insulin resistance. Blood glucose levels and adipose tissue were decreased, while insulin sensitivity and signaling were elevated (Hirosumi et al. 2002). More detailed research has shown that JNK1 induces the phosphorylation of IRS-1 residue S307 (corresponding to human S312), which inhibits insulin signaling (Lee et al. 2003). More recently, it has been shown that phosphorylation, even in other JNK pathways, could be promoted by endoreticulum stress (ER stress), which has been regarded to play an important role in metabolic regulation leading to the development of insulin resistance (Ozcan et al. 2004), impaired insulin biosynthesis (Kaneto et al. 2005b), and eventually to type 2 diabetes. JNK1 has also been found to decrease PDX-1 activity in terms of translocation and DNA binding, leading to down-regulation of insulin expression and β-cell secretory function (Kaneto et al. 2005a). JNK2 plays a role in the activation of T cells, which initiate autoimmune diseases, such as type 1 diabetes (Manning and Davis 2003). The JNK-signaling pathway mediates IL-1β-induced apoptosis in insulin-secreting cells, a mechanism relevant to the destruction of pancreatic β-cells in type 1 and 2 diabetes (Storling et al. 2005). Use of the JNK inhibitor JNK1 could prevent apoptosis, induced by activation of c-Jun NH2-terminal kinase (JNK) in isolated islets (Noguchi et al. 2005). Thus an increasing number of studies indicate that the JNK pathway plays a central role in pathogenesis of type 2 diabetes and may be a potential target for diabetes therapy.
Based on the above evidence, we hypothesized that MAP4K5 is a link between the TNF-α and JNK pathways and may be involved in type 2 diabetes pathogenesis. The case-controlled study indicates that in the studied population, the MAP4K5 -882 AA genotype was associated with a reduced risk of T2DM in the larger-waist-circumference population, but there was no trend toward a progressively reduced risk of T2DM from heterozygous to homozygous subjects for -882G/A. Thus the G allele is unlikely to be a factor that protects the central-obesity group of people from type 2 diabetes.
Though no differences in BMI and waist circumference were initially observed across the three genotype groups, after they were stratified by waist circumference, glucose level and AUCG were significantly lower for the AA genotype in the central-obesity group, showing the potential protective effect of the MAP4K5 -882 AA genotype against the risk of T2DM in the central-obesity group of people. With respect to the statistically higher insulin secretion index that the AA central-obesity group possessed, they were suggested to have a better pancreatic β-cell secretion function than the GA/GG central-obesity one. In addition, considering the insignificantly different insulin-resistance conditions among AA and GA/GG cases, it could be interpreted that in the central-obesity group, AA’s better insulin-secretion function could counteract the similar degree of insulin resistance from overburdened central adiposity, thus leading to the lower risk of type 2 diabetes in subjects carrying the AA genotype.
MAP4K5 -882 AA may act through the modulation of the MAP4K5 expression level since it may influence the activity of the TNF-α-induced JNK pathway, or through other pathways unknown as of yet. Alternatively, MAP4K5 -882G/A SNP might have no effect on the risk of type 2 diabetes per se, rather, it may serve as a marker of linkage disequilibrium with another causative genetic variant(s). This result should be confirmed by other independent studies in larger or other ethnic populations.
In conclusion, in the Han population in Shanghai, MAP4K5 -882G/A SNP was found to be associated with a reduced risk of type 2 diabetes in the central-obesity group. The G allele was related to higher glucose levels and higher risk of T2DM in this group. A larger population, with more SNPs of MAP4K5 and other genes in the TNF α/JNK pathway, as well as a function study on MAP4K5, are needed to see if the above hypothesis can be substantiated.
References
American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care 28:S37–S42
Cederholm J, Wibell L (1990) Insulin release and peripheral sensitivity at the oral glucose tolerance test. Diabetes Res Clin Pract 10:167–175
Chen Q, He G, Qin W, Chen QY, Zhao XZ, Duan SW, Liu XM, Feng GY, Xu YF, St Clair D, Li M, Wang JH, Xing YL, Shi JG, He L (2004) Family-based association study of synapsin II and schizophrenia. Am J Hum Genet 75(5):873–877
Christen U, Wolfe T, Mohrle U, Hughes AC, Rodrigo E, Green EA, Flavell RA, von Herrath MG (2001) A dual role for TNF-α in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J Immunol 166(12):7023–7032
Dalziel B, Gosby AK, Richman RM, Bryson JM, Caterson ID (2002) Association of the TNF–α -308 G/A promoter polymorphism with insulin resistance in obesity. Obes Res 10(5):401–407
Heijmans BT, Westendorp RG, Droog S, Kluft C, Knook DL, Slagboom PE (2002) Association of the tumour necrosis factor alpha -308G/A polymorphism with the risk of diabetes in an elderly population-based cohort. Genes Immun 3(4):225–228
Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS (2002) A central role for JNK in obesity and insulin resistance. Nature 420:333–336
International Diabetes Foundation (2005) Worldwide definition of the metabolic syndrome. Available at http://www.idf.org/webdata/docs/IDF_ syndrome and http://www.idf.org/webdata/docs/IDF_Metasyndrome_definition.pdf
Jaeschke A, Rincon M, Doran B, Reilly J, Neuberg D, Greiner DL, Shultz LD, Rossini AA, Flavell RA, Davis RJ (2005) Disruption of the Jnk2 (Mapk9) gene reduces destructive insulitis and diabetes in a mouse model of type I diabetes. Proc Natl Acad Sci USA 102(19):6931–6935
Kaneto H, Kawamori D, Nakatani Y, Gorogawa S, Matsuoka TA (2004) Oxidative stress and the JNK pathway as a potential therapeutic target for diabetes. Drug News Perspect 17(7):447–453
Kaneto H, Kawamori D, Matsuoka TA (2005a) Oxidative stress and pancreatic b-cell dysfunction. Am J Ther 12:529–533
Kaneto H, Matsuoka TA, Nakatani Y (2005b) Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med 83(6):429–439
Kim WH, Lee JW, Gao B, Jung MH (2005) Synergistic activation of JNK/SAPK induced by TNF-α and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal 17(12):1516–1532
Koch M, Rett K, Volk A, Maerker E, Haist K, Weisser M, Rettig A, Renn W, Haring HU (2000) The tumour necrosis factor alpha -238 G → A and -308 G→A promoter polymorphisms are not associated with insulin sensitivity and insulin secretion in young healthy relatives of type II diabetic patients. Diabetologia 43(2):181–184
Kubaszek A, Pihlajamaki J, Komarovski V, Lindi V, Lindstrom J, Eriksson J, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J,Uusitupa M, Laakso M (2003) Promoter polymorphisms of the TNF-α (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish diabetes prevention study. Diabetes 52(7):1872–1876
Lee YH, Giraud J, Davis RJ, White MF (2003) c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem 278:2896–2902
Li H, Groop L, Nilsson A, Weng J, Tuomi T (2003) A combination of human leukocyte antigen DQB1*02 and the tumor necrosis factor alpha promoter G308A polymorphism predisposes to an insulin-deficient phenotype in patients with type 2 diabetes. J Clin Endocrinol Metab 88(6):2767–2774
Manning MA, Davis RJ (2003) Targeting JNK for theraputic benifit: from junk to gold. Nat Rev Drug Discov 2:554
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Noguchi H, Nakai Y, Matsumoto S (2005) Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am J Transplant 5(8):1848–1855
Ozcan U, Cao Q, Yilmaz E (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306(15):457–461
Rasmussen SK, Urhammer SA, Jensen JN, Hansen T, Borch-Johnsen K, Pedersen O (2000) The -238 and -308 G→A polymorphisms of the tumor necrosis factor alpha gene promoter are not associated with features of the insulin resistance syndrome oraltered birth weight in Danish Caucasians. J Clin Endocrinol Metab 85(4):1731–1734
le Roith D, Zick Y (2001) Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care 24(3):588–597
Shi CS, Kehrl JH (2003) Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J Biol Chem 278(17):15429–15434
Shi CS, Tuscano J, Kehrl JH (2000) Adaptor proteins CRK and CRKL associate with the serine/threonine protein kinase GCKR promoting GCKR and SAPK activation. Blood 95(3):776–782
Shiau MY, Wu CY, Huang CN, Hu SW, Lin SJ, Chang YH (2003) TNF-α polymorphisms and type 2 diabetes mellitus in Taiwanese patients. Tissue Antigens 61(5):393–397
Storling J, Zaitsev SV, Kapelioukh IL (2005) Calcium has a permissive role in interleukin-1beta-induced c-jun N-terminal kinase activation in insulin-secreting cells. Endocrinology 146(7):3026–3036
Stumvoll M, Mitrakou A, Pimenta W, et al (2000) Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23:295–301
Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS (2001) IFN-gamma/TNF–α synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol 166(7):4481–4489
Acknowledgements
This study was supported in part by F. Hoffmann-La Roche, and the National Nature Science Foundation of China (No.30470817), 973 project of the China and Shanghai Leading Academic Discipline Project (Y0204).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, Y., Luo, T., Yang, J. et al. The -822G/A polymorphism in the promoter region of the MAP4K5 gene is associated with reduced risk of type 2 diabetes in Chinese Hans from Shanghai. J Hum Genet 51, 605–610 (2006). https://doi.org/10.1007/s10038-006-0402-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-006-0402-9
Keywords
This article is cited by
-
TS: a powerful truncated test to detect novel disease associated genes using publicly available gWAS summary data
BMC Bioinformatics (2020)
-
The mammalian family of sterile 20p-like protein kinases
Pflügers Archiv - European Journal of Physiology (2009)