Abstract
Genetic mechanisms are implicated as a cause of some male infertility, yet are poorly understood. Mouse meiotic mutant mei1 (meiosis defective 1) was isolated by a screening of infertile mice. Male mei1 mice have azoospermia due to meiotic arrest, and the mouse Mei1 gene is responsible for the mei1 phenotype. To investigate whether human MEI1 gene defects are associated with azoospermia by meiotic arrest, we isolated the human MEI1 cDNA based on the mouse Mei1 amino acid sequence. MEI1 is expressed specifically in the testis. Mutational analysis by direct sequencing of all MEI1 coding regions was performed in 27 men (13 European Americans, 13 Israeli and 1 Japanese) having azoospermia due to complete early meiotic arrest. This identified four novel, coding single-nucleotide-polymorphisms (cSNPs), i.e., SNP1 (T909G), SNP2 (A1582G), SNP3 (C1791A) and SNP4 (C2397T) in exons 4, 8, 9 and 14, respectively. Using these cSNPs, an association study was carried out between 26 non-Japanese patients with azoospermia and two sets of normal control men (61 normal European Americans and 60 Israelis). Consequently, SNP3 and SNP4 were shown to be associated with azoospermia among European Americans (P =0.0289 and P =0.0299 for genotype and allele frequencies at both the polymorphic sites, respectively), although no such association was observed among Israelis (P >0.05). Haplotype estimation revealed that the frequencies of SNP3–SNP4 (C–T), SNP3–SNP4 (A–C) and SNP3–SNP4 (A–T) were higher in the European American patients, and the frequency of SNP3–SNP4 (A–T) was also higher than in both control groups. These results suggest that MEI1 may play a role in meiosis during spermatogenesis, especially in European Americans.
Similar content being viewed by others
Introduction
Genetic causes of severe male infertility include chromosomal abnormalities such as Y-chromosome microdeletions and specific gene mutations in AZF, DAZ, RBMY, USP9Y and SYCP3 (Reijo et al. 1995; Elliott et al. 1997; Sun et al. 1999; Matzuk and Lamb 2002; Miyamoto et al. 2003). Since the Y-chromosome deletions explain only up to 21% of men with infertility (Nakamura et al. 2001), azoospermia in many infertile men may be caused by autosomal gene mutations. Genetic polymorphisms may also be factors susceptible to some forms of male infertility, albeit somewhat controversially, e.g., whether the CAG repeat of the human androgen-receptor gene is linked to male infertility (Dowsing et al. 1999).
Defective meiosis during spermatogenesis is one of the critical causes of azoospermia, although the details remain unknown. In sexually reproducing species, meiosis is a fundamental process that allows a genetic exchange between maternal and paternal genomes (Nasmyth 2002). The genetic regulation of meiosis in mammals is poorly understood compared to that in lower eukaryotes such as yeast. Several key genes expressed in mouse meiosis, including Dmc1, Fkbp6, Scp3 (Sycp3), Spo11, Msh4 and Msh5, have been identified by disruption experiments in embryonic stem (ES) cells (Yoshida et al. 1998; Pittman et al. 1998; Edelmann et al. 1999; Baudat et al. 2000; Kneitz et al. 2000; Romanienko and Camerini-Otero 2000; Yuan et al. 2000; Crackower et al. 2003). In addition, the mouse meiotic mutant mei1 (meiosis defective 1) was isolated by a screening of infertile mice generated by chemical mutagenesis in ES cells (Munroe et al. 2000). The male mice mutant for the Mei1 gene has small testes and lacks the epididymal sperm and postmeiotic cells. The seminiferous tubules of such mice contain spermatocytes arrested at the zygotene/pachytene stage of meiosis (Libby et al. 2003). In contrast, few genes essential to human meiosis are known.
In the present study, we isolated the human MEI1 cDNA using the deduced amino acid sequence of the mouse Mei1 cDNA, and analyzed a possible association of MEI1 mutations with azoospermia by meiotic arrest in man.
Materials and methods
Isolation of the human MEI1 cDNA and analysis of its expression in various tissues
The mouse Mei1 cDNA was isolated previously (Libby et al. 2003). Using the mouse Mei1 amino acid sequence (GenBank accession no. AY270177), we identified its homologous region in the human genome (GenBank BX391221). We designed a pair of primers (MEI1F2 and MEI1R2) in the human homologous region encompassing the putative introns, and carried out PCR on a human testis cDNA library (Clontech, Tokyo, Japan). The sequences (5′–3′) of the oligonucleotides used were as follows: MEI1F2, GCTGGAAGAAGCCATGCAGG; and MEI1R2, AGTCCGGTCCCTGGTCATTG. Semi-nested PCR was performed with MEI1F2/MEI1R3 and a 10-fold dilution of the first PCR product as a template. The other oligonucleotide (5′–3′) used was MEI1R3, TGCAGAACCTCCTGGTGCAG. The product from the semi-nested PCR was subcloned into a T-Easy vector (Promega, Madison, WI), and several representative clones were sequenced in both directions. The 5′- and 3′-RACE were performed with primers 5RAFUL1, 5RAFUL8, 3RA1, 3RA5, AP1 (Clontech) and AP2 (Clontech). Their sequences (5′–3′) were as follows: 5RAFUL1, GTACTGGCGATCAGACAGGAAGGCAAGG; 5RAFUL8, AAGGATGAGGAAGCTTCAGAGCCGTGGG; 3RA1, TGGATGCTGGAGAGAATTCCTTCCTCAG and 3RA5, TTTGGCTGACCTGTCTACCCTCTCGAAC. Both RACE products were also subcloned, and several representative clones were sequenced in both directions. The isolated full-length cDNA sequences were compared with human genomic sequences. All PCRs were carried out using an Advantage 2 PCR Kit (Clontech) under the following conditions and according to the manufacturer’s instructions: initial denaturation at 95°C for 150 s, followed by 32 cycles of denaturation at 95°C for 15 s, annealing and extension at 68°C for 90 s.
For expression analysis of MEI1, PCR of cDNA from various tissue types (spleen, thymus, prostate, testis, ovary, small intestine, colon, leukocyte, brain, heart, kidney, liver, lung, pancreas and placenta) purchased from Clontech was performed with EXP2F1 and EXP2R6 as primers. The sequences (5′–3′) of the primers were: EXP2F1, CTGGGAAGAGAGCAGCTATG; and EXP2R6, CTGCTGGGTGTGGTCTGATG. PCR conditions were initial denaturation at 95°C for 150 s, followed by 32 cycles of denaturation at 95°C for 15 s, annealing and extension at 68°C for 90 s using an Advantage 2 PCR Kit.
Patients and control individuals
All patients and donors participating in this study gave informed consent for molecular analysis of their blood samples, and the study protocol was approved by the Committee for the Ethical Issues on Human Genome and Gene Analysis, Tel Aviv Sourasky Medical Center and Kanazawa University. To test the hypothesis that human MEI1 gene mutations are associated with human azoospermia, we screened 27 patients diagnosed as having azoospermia due to complete meiotic arrest. Genomic DNA from 13 of these patients was obtained from the NIH funded tissue bank at Baylor College of Medicine with the patients’ written informed consent and the full oversight of the Institutional Review Boards for the Protection of Human Subjects at Baylor College of Medicine. These men underwent clinical evaluations for diagnosis and treatment of their azoospermia by an expert diagnostician, Dr. Larry Lipshultz. They provided an extensive history and underwent a physical examination, as well as state-of-the-art andrology testing (showing a normal karyotype by high resolution banding chromosome analysis and by testing for Y-chromosome microdeletions). Consequently, azoospermia was diagnosed to be idiopathic in all cases. Of the 27 patients, 13 were European Americans, 13 were from Israel and the remaining 1 was from Japan, and all had normal karyotypes and no Y-chromosome microdeletions. Pathological examination of bilateral testicular biopsy specimens from 26 (13 European Americans and 13 Israelis) of the 27 men showed an early maturation arrest testicular phenotype (the most mature spermatogenic cell type present was the spermatocyte), which was consistent with that seen in mei1 mutant mice. Sixty-one and 60 healthy and pregnancy-proven fertile men (European American and Israeli, respectively) were used as normal control individuals.
MEI1 mutation screening
To test the hypothesis that MEI1 gene mutations are associated with human non-obstructive azoospermia, we screened 27 patients for mutations in MEI1. Genomic DNA was obtained from peripheral blood lymphocytes using a Qiagen Blood and Cell Culture DNA Midi Kit (Qiagen, Hilden, Germany). After all exon–intron borders were identified by comparison between the full-length cDNA and genomic sequences (NT_007758.10), all MEI1 coding regions and intronic sequences adjacent to exons of the patients were analyzed by direct sequencing. Nested or semi-nested PCRs were performed using primers for each intronic region (Table 1) and 10-fold diluted first PCR products as templates. PCR was performed in a final volume of 25 μl, consisting of genomic DNA (10 ng), dNTP (0.32 mM each) (TaKaRa, Shiga, Japan), each primer (0.2 mM), Taq polymerase (0.625 U) (Roche, Tokyo, Japan) and reaction buffer containing MgCl2(Roche). Nested and semi-nested PCRs were carried out for 20 cycles under the same conditions above but with 2 μl of 10-fold diluted first PCR products as templates, using a programmable PC 960G gradient thermal cycler (Cosmo Bio, Tokyo, Japan) using the following PCR conditions: initial denaturation at 95°C for 150 s, followed by 32 cycles of denaturation at 95°C for 30 s, annealing at (primer Tm−5°C) for 90 s and extension at 72°C for 90 s. PCR products were purified using a QIAquick PCR Purification kit (Qiagen), and direct sequencing of each product was carried out in both directions.
Genotyping and statistical analyses
Single-locus analysis
To investigate the role of MEI1 polymorphisms in azoospermia, 26 patients (13 European Americans and 13 Israelis) were genotyped for polymorphic alleles, and compared to the genotype and allele frequencies in the normal control men. To match ethnic populations, the single Japanese patient was excluded from the study. Fisher’s exact test was used to determine the significance of differences. P values of 0.05 or less were considered statistically significant. The Hardy–Weinberg equilibrium (HWE) was tested using a commercial program (SNPAlyze ver 5.0 Standard; Dynacom, Chiba, Japan).
Pairwise locus disequilibrium analysis
The measure of linkage disequilibrium (LD) known as D’ (Lewontin 1988), which is corrected for allele frequencies of loci, was computed for allele at pairs of SNP loci using the 26 patients. Tests of departures from linkage equilibrium were performed using the composite test for the overall SNPs. P values were determined via χ2 approximation. As described above, significance was determined at the P =0.05 level. These calculations were performed using a commercial program (SNPAlyze ver 5.0 Standard).
Haplotype frequency estimation
Haplotype frequencies were estimated by the method of maximum likelihood from genotype data through the use of the expectation–maximization (E–M) algorithm under the assumption of HWE (Excoffier and Slatkin 1995). Haplotype-based hypothesis tests focused on the case and control groups. Haplotypes of SNP were assessed using the EH package soft (http://linkage.rockefeller.edu/soft/). Chi-square statistics were derived from a series of simple 2×2 tables based on the frequency of each haplotype versus all others combined between the case and control groups. P values were determined via χ2 approximation. Significance was determined at the P =0.05 level.
Results
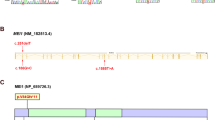
Based on the amino acid sequence deduced from the mouse Mei1 cDNA, we have identified the human MEI1 cDNA. Comparison of the cDNA and its corresponding genomic sequence revealed that MEI1 is located to chromosome 22q13.2, consists of 16 exons encompassing over 47.1 kb in the genome, with an open reading frame (ORF) from nt 634 to 2,562 (Fig. 1). MEI1 has at least two alternative transcripts of 2,714 and 2,609 bp (annotated as AY952376 and AY952377 in GenBank, respectively), encoding proteins of 642 and 607 amino acids, respectively. The shorter 2,609-bp cDNA lacks exon 7 as a result of alternative splicing. Furthermore, the 642 amino acid protein shares 77% homology with the mouse Mei1 protein (Fig. 2) and the cDNA in the coding region shares 61% homology with the mouse Mei1 cDNA. However, no previously known functional domains could be found in human MEI1. Analysis of expression patterns of MEI1 in various adult tissues revealed that it was predominately expressed in the testis and weakly in the spleen and thymus (Fig. 3).
Gene structure and four novel coding single-nucleotide-polymorphism (cSNP) sites in the human MEI1 gene. The gene consists of 16 exons, and alternative splicing events generate two mRNA products. The shorter cDNA lacks exon 7. Arrows indicate four novel SNPs (SNP1, SNP2, SNP3 and SNP4), the start codon and the stop codon
RT–PCR analysis of human MEI1 cDNA using primers EXP2F1 and EXP2R6. Expression patterns of MEI1 in 15 human adult tissue cDNA samples were examined by PCR (upper panel). Two clear bands were detected in the testis and very weak bands were present in spleen and thymus. The upper and lower bands in the testis lane indicate the longer and shorter cDNAs, respectively. G3PDH was used as a positive control (lower panel)
Mutation analysis of MEI1 revealed four nucleotide changes among the 27 patients, i.e., T909G in exon 4, A1582G in exon 8, C1791A in exon 9 and C2397T in exon 14. As the four coding single nucleotide polymorphisms (cSNPs) were hitherto undescribed or have not been registered in the NCBI database, they are novel cSNPs (Table 2). Among SNP1 (T909G), SNP2 (A1582G), SNP3 (C1791A) and SNP4 (C2397T), the latter three SNPs were observed in the heterozygous state in all but the Japanese patient and SNP2 is non-synonymous (Thr–Ala). We did not find any of these SNPs in the Japanese patient.
Genotyping for MEI1 SNP alleles among the 26 patients (13 European Americans and 13 Israelis) and 61 control individuals (European Americans) revealed that the genotype distribution and the allele frequency of SNP3 and SNP4 were significantly different between the two groups (Table 2). At the 1791A/C site (SNP3), the proportions of AA homozygote/AC heterozygote/CC homozygote in the patient and control groups were 0.00/0.154/0.846 and 0.000/0.000/1.000, respectively (P =0.0067). The allele frequencies for 1791A/C in the two groups were 0.077/0.923 and 0.00/1.000, respectively, and were significantly different (P =0.0073). Likewise, at the 2397T/C site (SNP4), the proportions of the respective zygosity in the two groups were 0.000/0.115/0.885 and 0.00/0.00/1.000 (P =0.0245), and the allele frequencies were 0.057/0.943 and 0.000/1.000 (P =0.0256). The most common genotypes for SNP3 and SNP4 in both groups were 1791C/C and 2397C/C. However, AC heterozygotes and TC heterozygotes were strikingly higher at the SNP3 and SNP4 sites in the patient group, respectively. There were no statistical differences for SNP1 and SNP2 in the two groups (P >0.05 for both SNPs). Tests of HWE carried out for all SNPs among patients revealed that SNP2 of patients showed a significant deviation from HWE (P <0.05).
We next analyzed a possible association of the MEI1 SNPs with azoospermia separately among European Americans and among Israelis (Table 3). At the SNP3 site, the proportions of AA homozygote/AC heterozygote/CC homozygote in the 13 European American patients and their 61 controls were 0.00/0.154/0.846 and 0.000/0.000/1.000, respectively (P =0.0289), and the frequencies for alleles A/C were 0.077/0.923 and 0.00/1.00, respectively, showing a significant difference (P =0.0299). Similarly, at the SNP4 site, the proportions of the respective zygosity in the two groups were 0.000/0.154/0.846 and 0.000/0.000/1.000 (P =0.0289), and the allele frequencies were 0.077/0.923 and 0.00/1.00 (P =0.0299). However, no such association at the two polymorphic sites was observed between the 13 Israelis and their 60 control individuals (P >0.05).
Haplotype analysis revealed that haplotype frequencies estimated for all four polymorphisms in the groups were close to each other, with no significant differences (data not shown). Haplotype estimation and LD analysis revealed different distributions of the haplotypes with SNP3 and SNP4 (Tables 4, 5). The SNP3–SNP4 (C–T), SNP3–SNP4 (A–C) and SNP3–SNP4 (A–T) haplotypes were revealed to be significantly more frequent in the European American patient group than in the European American control group. The SNP3–SNP4 (A–T) haplotype was also revealed to be significantly more frequent in the Israeli patient group than in the Israeli control group.
Discussion
We have isolated the human MEI1 cDNA, which shares 61 and 77% homology at the nucleotide and amino acid levels, respectively, to the mouse Mei1 cDNA. The longest ORF of the mouse Mei1 comprises 2,685 bp and is predicted to encode a protein of 894 amino acids. Two alternative MEI1 transcripts consisting of 2,714 and 2,609 bp encode proteins of 642 and 607 amino acids, respectively, as in the mouse Mei1 gene (Libby et al. 2003). The human MEI1 is not assigned to the Y chromosome but is located to 22q13.2. The predominant expression of MEI1 in the testis is consistent with its putative role in spermatogenesis, as seen in the mouse Mei1 gene (Libby et al. 2003). Positional cloning showed that mouse Mei1 is responsible for the mutant mei1 phenotype. The mei1 mice lack the first 58 bp in exon 12 or entirely skip exon 12 of Mei1, resulting in a frameshift leading to a predicted truncated Mei1 protein. Male mice with such a mutated Mei1 show spermatocytes arrested at meiosis, and the RAD51 protein does not load onto chromosomes bearing mutated Mei1, suggesting that there is a defect either in recombinational repair or in the production of double-strand breaks (DSBs) that require such repair (Libby et al. 2003). Recent studies on meiosis in (Mei1−/−) and (Dmc1−/−) mice of both sexes have demonstrated that their phenotypes are identical to those of Mei1−/− mice (Reinholdt and Schimenti 2005). Therefore, Mei1 can be positioned upstream of Dmc1 in the genetic pathway that operates during mouse meiosis. Further analysis is needed to determine the relationship between MEI1 and DMC1 in man.
We have identified four novel cSNPs in the MEI1 gene. The present association study has revealed that the genotype distributions for SNP3 (A/C) and SNP4 (T/C) are significantly different between the European American azoospermic patients and their controls: 0.154/0.846 vs 0.000/1.000 for AC heterozygotes/CC homozygotes at the SNP3 site; and 0.154/0.846 vs 0.000/1.000 for TC heterozygotes/CC homozygotes at the SNP4 site, respectively (P <0.05). Likewise, the frequencies of alleles A/C at SNP3 were 0.077/0.923 and 0.00/1.000; and those of alleles T/C at SNP4 were 0.077/0.923 vs 0.000/1.000 in the patients and controls, respectively (P <0.05). These findings suggest that allele C at nucleotide 1,791 in exon 9 and allele C at nucleotide 2,397 in exon 14, or their flanking regions, may play a role in the disruption of spermatogenesis in the European American patients, although the number of patients analyzed was not large enough to allow a definitive conclusion to be drawn; no such association was found in Israeli patients.
HWE tests performed for the four cSNPs in European American and Israeli patients ruled out the equilibrium of SNP2 by its P value. This deviation is most likely due to the small sample size in the present study. We performed haplotype analysis on the synonymous SNPs identified as well as their combinations, and found no significant difference between the haplotype frequencies estimated for all four polymorphisms between the patient and control groups. We then performed LD analysis using pairs of SNPs. Consequently, we detected three pairs of SNPs (SNP2–SNP3, SNP2–SNP4 and SNP3–SNP4) with LD values of 1.00 and with P values of <0.05 by χ2 tests. We carried out haplotype analysis on the SNP3–SNP4 pair containing statistical differences in genotype and allelic levels. All analyses were performed both in European American and Israeli patients. Haplotype analysis demonstrated that three haplotypes, SNP3–SNP4 (C–T), SNP3–SNP4 (A–C) and SNP3–SNP4 (A–T), were markedly associated with azoospermia among the European American patients. Furthermore, such an association of a SNP3–SNP4 (A–T) haplotype was also found among the Israeli patients.
In vitro fertilization (IVF) has been proven to be an efficient way to resolve infertility due to female factors (Edwards et al. 1980), but it has not been so effective for problems due to severe oligospermia in the male partner (Devroey and Van Steirteghem 2004). Although testicular sperm extraction (TESE)–intracytoplasmic sperm injection (ICSI) is now available for patients with azoospermia, it cannot help patients lacking spermatozoa in their testes due to a complete failure in spermatogenesis. Therefore, treatment for infertility due to non-obstructive azoospermia is an important immediate goal in assisted reproductive technology (ART).
In conclusion, this is the first report showing that MEI1 SNPs may predispose men to a defect in spermatogenesis, although the mechanism by which these SNPs result in azoospermia remains uncertain. Our results may also advance a better understanding of the molecular basis of early meiotic arrest as a cause of non-obstructive azoospermia. It remains to be confirmed whether the association is seen in larger sample numbers and in similar patients from other ethnic groups, although men with azoospermia caused by meiotic arrest are very rare.
References
Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6:989–998
Crackower MA, Kolas NK, Noguchi J, Sarao R, Kikuchi K, Kaneko H, Kobayashi E, Kawai Y, Kozieradzki I, Landers R et al (2003) Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science 300:1291–1295
Devroey P, Van Steirteghem A (2004) A review of ten years experience of ICSI. Hum Reprod 10:19–28
Dowsing AT, Yong EL, Clark M, McLachlan RI, de Kretser DM, Trounson AO (1999) Linkage between male infertility and trinucleotide repeat expansion in the androgen-receptor gene. Lancet 354:640–643
Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R (1999) Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet 21:123–127
Edwards RG, Steptoe PC, Purdy JM (1980) Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynecol 87:737–756
Elliott DJ, Millar MR, Oghene K, Ross A, Kiesewetter F, Pryor J, McIntyre M, Hargreave TB, Saunders PT, Vogt PH et al (1997) Expression of RBM in the nuclei of human germ cells is dependent on a critical region of the Y chromosome long arm. Proc Natl Acad Sci USA 94:3848–3853
Excoffier L, Slatkin M (1995) Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol 12:921–927
Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou H Jr, Kolodner RD, Kucherlapati R, Pollard JW, Edelmann W (2000) MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev 14:1085–1097
Lewontin RC (1988) On measures of gametic disequilibrium. Genetics 120:849–852
Libby BJ, Reinholdt LG, Schimenti JC (2003) Positional cloning and characterization of Mei1, a vertebrate-specific gene required for normal meiotic chromosome synapsis in mice. Proc Natl Acad Sci USA 100:15706–15711
Matzuk MM, Lamb DJ (2002) Genetic dissection of mammalian fertility pathway. Nat Med 8[Suppl 1]:S41–S49
Miyamoto T, Hasuike S, Yogev L, Maduro MR, Ishikawa M, Westphal H, Lamb DJ (2003) Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet 362:1714–1719
Munroe RJ, Bergstrom RA, Zheng QY, Libby B, Smith R, John SW, Schimenti KJ, Browning VL, Schimenti JC (2000) Mouse mutants from chemically mutagenized embryonic stem cells. Nat Genet 24:318–321
Nakamura Y, Kitamura M, Nishimura K, Koga M, Kondoh N, Takeyama M, Matsumiya K, Okuyama A (2001) Chromosomal variants among 1790 infertile men. Int J Urol 8:49–52
Nasmyth K (2002) Segregating sister genomes: the molecular biology of chromosome separation. Science 297:559–565
Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, Handel MA, Schimenti JC (1998) Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell 1:697–705
Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O et al (1995) Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 10:383–393
Reinholdt LG, Schimenti JC (2005) Mei1 is epistatic to Dmc1 during mouse meiosis. Chromosoma 114:127–134
Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6:975–987
Sun C, Skaletsky H, Birren B, Devon K, Tang Z, Silber S, Oates R, Page DC (1999) An azoospermic man with a de novo point mutation in the Y-chromosomal gene USP9Y. Nat Genet 23:429–432
Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T (1998) The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell 1:707–718
Yuan L, Liu JG, Zhao J, Brundell E, Daneholt B, Hoog C (2000) The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell 5:73–83
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (Nos. 16,390,471, 15,591,725 and 16,790,934) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, from the Ministry of Health, Labour and Welfare of Japan and from the Kamizawa Medical and Akiyama foundation. Patient and control samples were obtained from Male Infertility Tissue Bank, and supported in part by P01 HD 36289 to D.J.L. from the National Institutes of Health of the USA. The authors would like to thank Dr. Larry I. Lipshultz for his expert evaluation of the patients seen at Baylor College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, H., Miyamoto, T., Yogev, L. et al. Polymorphic alleles of the human MEI1 gene are associated with human azoospermia by meiotic arrest. J Hum Genet 51, 533–540 (2006). https://doi.org/10.1007/s10038-006-0394-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-006-0394-5
Keywords
This article is cited by
-
Transcriptome profiling of developing testes and spermatogenesis in the Mongolian horse
BMC Genetics (2020)
-
Genetic defects in human azoospermia
Basic and Clinical Andrology (2019)
-
New insights into the genetics of spermatogenic failure: a review of the literature
Human Genetics (2019)
-
The use of fluorescence in situ hybridization analysis on sperm: indications to perform and assisted reproduction technology outcomes
Journal of Assisted Reproduction and Genetics (2019)
-
Genome-wide association study identifies a novel locus for cannabis dependence
Molecular Psychiatry (2018)