Abstract
Cytokines, having central functions in immunological and inflammatory process, are always expected to play important roles in the pathogenesis of various diseases, such as asthma. Genetic polymorphisms of those cytokine and cytokine receptor genes are the focus of genetic association studies. In an effort to identify gene(s) whose variant(s) are involved in the development of asthma, we examined the genetic effects of 19 single nucleotide polymorphisms in eight cytokine and cytokine receptor genes, including IL1A, IL1B, IL2, IL3, IL4, IL8, IL10, and IL5RA, on asthma and atopy. Nineteen single nucleotide polymorphisms in eight cytokine and cytokine receptor genes were genotyped using the single-base extension method in a Korean asthma cohort (n=723). Logistic regression and multiple regressions were used for statistical analyses controlling for smoking, age, and gender as covariables. Genetic association analysis of polymorphisms revealed that one exonic (exon 1), IL3+79T>C (Ser27Pro), showed significant association with the risk of asthma and atopy. The Pro allele had shown dominant and protective effects on development of asthma in nonatopic subjects (P=0.002) and also showed significant association with the risk of atopy in normal control subjects (P=0.007). This information about the genetic association of important genes with asthma might provide valuable insights into strategies for the pathogenesis of asthma and atopy.
Similar content being viewed by others
Introduction
Asthma is a common and heterogeneous respiratory disease characterized by reversible airways obstruction caused by chronic inflammation of the airways. Bronchial hyperresponsiveness is a characteristic feature of asthma, and serum IgE levels are closely associated with asthma development. The development of asthma is determined by the interaction between host genetic susceptibility and a variety of environmental exposures (Ahmadi and Goldstein 2002; Burrows et al. 1989; Kim et al. 1999; Koh et al. 2000). Asthma is recognized as a T-helper type 2 (Th2) disease with a particular profile of cytokine release, including IL4 and IL5. However, increasing evidence indicates that other cytokines, which were classically considered to belong to Th1-type profiles, are also associated with the inflammatory response that characterizes human asthma (Chung and Barnes 1999). Cytokines play an important role in the coordination and persistence of the inflammatory process in chronic inflammation of the airways in asthma and many other diseases. Chronic and acute inflammatory changes observed in the asthmatic airway could result from excessive release of many types of cytokines (Broide et al. 1992; Chung and Barnes 1999; Robinson et al. 1992).
Several interleukins have been reported to be involved in asthma through the pathological and immunological pathways as lymphokines, proinflammatory cytokines, and anti-inflammatory cytokines (Bagley et al. 1997; Chouchane et al. 1999; Chung and Barnes 1999; Karjalainen et al. 2003a; Renauld 2001). The IL1 gene complex is involved in the regulation of IgE-mediated atopic reactions and eosinophil accumulation in vivo (Chung and Barnes 1999). IL2 enhances the production of GM-CSF in peripheral blood mononuclear cells from asthmatics and the production of IL5 from T cells in hypereosinophilic syndrome patients as a potent chemoattractant for eosinophil (Enokihara et al. 1989; Nakamura et al. 1993; Rand et al. 1991). IL3, together with IL5 and GM-CSF, are important modulators of eosinophilia and eosinophil function. The differentiation, migration, and pathological effects of eosinophils may occur through the effects of GM-CSF, IL3, and IL5. IL8 possesses chemotactic activity for primed eosinophil and induces accumulation of eosinophils (Shute et al. 1997; Warringa et al. 1991). IL4 and IL10 play an important role in modulating total serum IgE. IL4 has been shown to play a crucial role in the pathogenesis of allergic disease including bronchial asthma, and IL4 increases airway responsiveness by recruiting eosinophils into the airway in patients with allergic bronchial asthma. In addition, IL10 inhibits eosinophil survival and IL4 induces IgE synthesis (Jeannin et al. 1998; Shi et al. 1998). These functions of the two genes are deeply associated with their polymorphisms.
In the current research, we examined several genetic polymorphisms in important cytokine and receptor genes (IL1A, IL1B, IL2, IL3, IL4, IL8, IL10, and IL5RA) using data from a Korean asthma study that was established recently.
Materials and methods
Subjects
Subjects were recruited from the asthma genome research center that consists of four tertiary hospitals in Korea (Soonchunhyang University Hospital, Ajuo University Hospital, Choong-Ang University Hospital, and Ulsan University Hospital). Ethical approvals were obtained from the institutional review board of each hospital. All patients had met the definition of asthma by the American Thoracic Society (1987). Normal subjects were recruited from spouses of the patients and from the general population who answered negatively to a screening questionnaire for respiratory symptoms and had FEV1 greater than 75% predicted, PC20 methacholine greater than 10 mg/ml, and normal findings on a simple chest radiogram. Total IgE and specific IgE to Dermatophagoides farinae (Df) and D. pteronyssinus (Dp) were measured by the CAP system (Pharmacia Diagnostics, Sweden). Twenty-four common inhalant allergens, including dust mites (D. farinae and D. pteronyssinus), car fur, dog fur, cockroaches, grass, tree pollens, and ragweed, were used for the skin-prick test. Atopy was defined as having wheal reaction by allergen equal to or greater than that by histamine (1 mg/ml) or 3 mm in diameter and/or positive response of specific IgE to Dp and Df. Clinical parameters are summarized in Table 1.
Genotyping by single base extension (SBE) and electrophoresis
Primer extension reactions were performed with SNaPshot ddNTP Primer Extension Kit (Applied Biosystems, Foster City, CA, USA) (Table 2). To clean up the primer extension reaction, one unit of SAP was added to the reaction mixture, and the mixture was incubated at 37°C for 1 h, followed by 15 min at 72°C for enzyme inactivation. The DNA samples, containing extension products and Genescan 120 Liz size standard solutions, were added to Hi-Di formamide (Applied Biosystems) according to the recommendation of the manufacturer. The mixture was incubated at 95°C for 5 min followed by 5 min on ice, and electrophoresis was performed by ABI Prism 3100 Genetic Analyzer. The results were analyzed using the program of ABI Prism GeneScan and Genotyper (Applied Biosystems).
Statistical analysis
We examined Lewontin’s D′ (|D′|) and LD coefficient r2 between all pairs of biallelic loci (Hedrick and Kumar 2001; Hedrick 1987). Haplotypes and their frequencies were inferred using the algorithm developed by Stephens et al. (2001). Logistic regression models were used for calculating odds ratios (95% confidential interval) and corresponding P values for single nucleotide polymorphism (SNP) sites and haplotypes controlling for age and gender as covariates.
Results
Nineteen SNPs in cytokine and cytokine receptor genes, including IL1A, IL1B, IL2, IL3, IL4, IL8, IL10, and IL5RA, were examined in a Korean asthma study (n=723). The allele frequencies in asthmatic and normal control subjects are shown in Table 5. Linkage disequilibria among SNPs in each gene were measured by calculating Lewontin’s D′ and r2 values (Table 3). The SNPs in absolute LD (|D′|=1 and r2=1): IL1A−1203 and IL1A−889 [=IL1A+4845 (Arg135Ser)], IL3−68 [=IL3+79 (Ser27Pro)], and IL10−819 (=IL10−592) were not used for further analysis (Table 3). Haplotypes in each gene were constructed as follows: three in IL1A, three in IL1B, three in IL2, two in IL3, four in IL4, four in IL8, and four in IL10 (Table 4). Haplotypes that had frequencies less than 3% and/or were almost equivalent to single SNPs were excluded in statistical analysis to avoid redundant statistical tests.
Genotype distribution of each SNP and common haplotypes of eight genes were compared (1) between asthma patients and the normal controls, and (2) between atopic and nonatopic subjects using logistic regression models controlling for age, gender, and smoking as covariates (Table 5). Among 19 polymorphisms tested in eight genes, one exonic (exon 1 in IL3) polymorphism that changes the amino acid Serine to Proline (IL3+79T>C; Ser27Pro) revealed significant association with the risk of asthma, whereas all other polymorphisms showed no noticeable difference in allele frequency. The positive associations of IL10 (IL10−592A>C) and IL4 (IL4−589T>C) polymorphisms with the severity or risk of asthma reported in Taiwanese (Hang et al. 2003), Caucasians (Hobbs et al. 1998), and Japanese (Noguchi et al. 2001, 1998) populations were not detected in the Korean population in this study (Table 5). The minor allele (Pro) of IL3+79T>C (Ser27Pro) showed a lower frequency in the asthmatic patient than in the normal control group (0.46 versus 0.54, P=0.01), and this association was even clearer in the nonatopic population (0.41 versus 0.62, P=0.0003).
The genetic influences of IL3 Ser27Pro (T>C) were further analyzed in four alternative models (reference, codominant, dominant, and recessive models) and subgroup analyses according to atopic status and diagnosis of subjects (Table 6). The reference analysis revealed that the protective genetic effects of Pro might be similar between heterozygotes and homozygotes (OR=0.80 versus 0.70 in all subjects and OR=0.34 versus 0.37 in nonatopic subjects), although much stronger associations were apparent in homozygotes than heterozygotes (P=0.40 versus 0.01 in all subjects and P=0.02 versus 0.0001 in nonatopic subjects). When considering referent analysis results, the effects of the Pro allele in the nonatopic group might be dominant, although no significant associations were found in the atopic group. The lack of a positive signal in the atopic group, as well as the stronger (P=0.02–0.0001) and more protective (OR=0.25–0.41) effects in the nonatopic subgroup, suggest that the association signal found in all subjects likely comes from the nonatopic subjects. Association analyses of the Pro allele with atopy were also performed. Although no positive signals were detected in all subjects and asthmatic patients, significant associations with atopy were apparent in normal controls.
In conclusion, it could be suggested that the Pro allele of the IL3 Ser>Pro locus had a dominant and protective genetic effect on the risk of asthma development in nonatopic subjects rather than atopic subjects. It was also protective against atopy in normal controls rather than in asthmatic patients.
Discussion
Asthma is a polygenic and common respiratory disease involving genetic and environmental factors. Identification of genetic polymorphisms, which are involved in the development of asthma, might be clinically useful for both identifying patients at risk and preventing the occurrence of the disease (Townley et al. 1986). Cytokines are extracellular-signaling proteins. They act on target cells to cause a wide array of cellular functions including activation, proliferation, immunomodulation, and release of other cytokines or mediators. Several researchers have reported the functions of cytokines in asthma as well as association with their polymorphisms with asthma (Chouchane et al. 1999; Chung and Barnes 1999; Hobbs et al. 1998; Walley and Cookson 1996; Zhang et al. 2002).
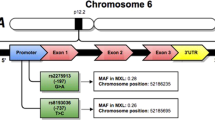
It has been suggested that one or more genes on chromosome 5q31-q33, including IL3, IL4, IL5, IL9, IL13, and GM-CSF gene cluster, might cause susceptibility to asthma, and their polymorphisms may be associated with the risk of asthma (Postma et al. 1995). The IL3 lies on chromosome 5q31.1, together with GM-CSF, IL4, IL5, IL9, and macrophage colony-stimulating factor. IL3 is mainly produced from activated T cells and mast cells (Arai et al. 1990; Fung et al. 1984). An increase in number of cells expressing IL3 mRNA has been reported in patients with asthma (Robinson et al. 1992), and IL3 caused an increase in the number of mast cells and eosinophils around the airways (Du et al. 1999). Also, eosinophil has been associated with asthma and allergic diseases since increased numbers of cells are present in blood and airway samples, and these numbers can be related to disease severity (Bousquet et al. 1990; Gleich 1990).
The rolls of IL3 in asthma have not been relatively studied as yet. However, the increase in eosinophils in mild and allergen-induced asthma patients occur independent of IL3 (Du et al. 1999; Woolley et al. 1996). Because of the ability to accumulate eosinophils, IL3 has been implicated in chronic inflammatory conditions mediated by eosinophils, of which allergic inflammation such as asthma is one of the most studied. In bronchial asthma, eosinophils have long been recognized to play a central role as judged by their accumulation in the bronchoalveolar lavage fluid and presence in the sputum of eosinophil cationic proteins believed to contribute to local tissue damage (Owen et al. 1987). Moreover, IL3 has been linked to several other pathologic features of asthma including subepithelial fibrosis, mucus hyper-secretion, and eotaxin production (Zhu et al. 1999). Despite the potential importance of IL3 in the pathogenesis of asthma and allergic pathogenesis, the genetic influence of IL3 gene polymorphisms on asthma has not yet been reported.
In this study, we demonstrated that IL3+79T>C (Ser27Pro), which is in absolute LD with one promoter SNP (IL3−68T>C), was significantly associated with nonatopic bronchial asthma and also with atopy in the nonasthmatic population. Although asthma is a multifactorial condition, the strongest risk factor in the etiology of asthma is atopy. The atopic individuals have significantly greater probability of developing asthma, and people with a family history of atopic disease(s) are at greatest risk of asthma. The association of the IL3 polymorphism with the risk of asthma in nonatopic subjects in this study might present clues of different genetic etiology and pathogenesis of intrinsic from extrinsic asthma. Similarly, genetic association with the risk of atopy in normal controls might also provide the different genetic background of atopy other than asthma. Recently, one positive correlation between IL3 polymorphisms and rheumatoid arthritis (RA) has been reported (Yamada et al. 2001). Because no functional studies were performed on promoter polymorphisms (IL3−68T>C) and/or missense polymorphism (IL3+79T>C [Ser27Pro]), which were in absolute LD, it is not clear which site caused the phenotypic difference. However, the decreased risk of asthma and atopy might possibly come from IL3+79T>C (Ser27Pro) because of several plausible reasons, e.g., (1) amino acid substitution to proline could cause the conformational change of N-terminal subdomain and consequent alteration of function, (2) there was no putative transcription factor-binding motifs on and adjacent to IL3−68T>C (TFSEARCH Searching Transcription Factor Binding Sites V1.3. [http://molsun1.cbrc.aist.go.jp/research/db/TFSEARCH.html], putative score >0.85). Further study would be needed to elucidate the functions of the variants more extensively.
Although the sources of discrepancies among current study and previous reports—including the positive associations of IL10 (IL10−592A>C) and IL4 (IL4−589T>C) polymorphisms with the severity or risk of asthma reported in Taiwanese (Hang et al. 2003), Caucasians (Hobbs et al. 1998), and Japanese (Noguchi et al. 2001, 1998) populations were not detected in the Korean population in this study (Table 5)—might be hard to clarify, several factors could provide plausible explanations, e.g., (1) possible genetic difference among different ethnic groups, (2) small size of this study [especially normal controls (n=171)], and (3) different study designs and possible biases in recruiting case and controls among studies.
In summary, we have examined the genetic association of several interleukins, including IL1A and IL1B (proinflammatory cytokines), IL2, IL3, IL4 (lymphokines), IL8 (chemokines), IL10 (anti-inflammatory cytokines), and IL5RA, with the risk of asthma and atopy. Genetic association study revealed that the ser allele of IL3+79T>C (Ser27Pro) locus had a dominant and protective genetic effect on the risk of asthma development in nonatopic subjects rather than atopic subjects. It also was protective to development of atopy in normal controls compared to asthmatic patients. The results of this study could be helpful in understanding the important function of IL3 in asthma and atopy development and also could be a useful target for drug development to treat asthma and/or atopy.
References
Ahmadi KR, Goldstein DB (2002) Multifactorial diseases: asthma genetics point the way. Curr Biol 12:R702–R704
American Thoracic Society (1987) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis 136:225–244
Arai KI, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T (1990) Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem 59:783–836
Bagley CJ, Woodcock JM, Stomski FC, Lopez AF (1997) The structural and functional basis of cytokine receptor activation: lessons from the common beta subunit of the granulocyte-macrophage colony-stimulating factor, interleukin-3 (IL-3), and IL-5 receptors. Blood 89:1471–1482
Beghe B, Barton S, Rorke S, Peng Q, Sayers I, Gaunt T, Keith TP, Clough JB, Holgate ST, Holloway JW (2003) Polymorphisms in the interleukin-4 and interleukin-4 receptor alpha chain genes confer susceptibility to asthma and atopy in a Caucasian population. Clin Exp Allergy 33:1111–1117
Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P et al (1990) Eosinophilic inflammation in asthma. N Engl J Med 323:1033–1039
Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI (1992) Cytokines in symptomatic asthma airways. J Allergy Clin Immunol 89:958–967
Burchard EG, Silverman EK, Rosenwasser LJ, Borish L, Yandava C, Pillari A, Weiss ST, Hasday J, Lilly CM, Ford JG, Drazen JM (1999) Association between a sequence variant in the IL-4 gene promoter and FEV(1) in asthma. Am J Respir Crit Care Med 160:919–922
Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG (1989) Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med 320:271–277
Chouchane L, Sfar I, Bousaffara R, El Kamel A, Sfar MT, Ismail A (1999) A repeat polymorphism in interleukin-4 gene is highly associated with specific clinical phenotypes of asthma. Int Arch Allergy Immunol 120:50–55
Chung KF, Barnes PJ (1999) Cytokines in asthma. Thorax 54:825–857
Cui T, Wu J, Pan S, Xie J (2003) Polymorphisms in the IL-4 and IL-4R [alpha] genes and allergic asthma. Clin Chem Lab Med 41:888–892
Du T, Martin JG, Xu LJ, Powell WS, Renzi PM (1999) IL-3 does not affect the allergic airway responses and leukotriene production after allergen challenge in rats. Eur Respir J 13:970–975
Elliott K, Fitzpatrick E, Hill D, Brown J, Adams S, Chee P, Stewart G, Fulcher D, Tang M, Kemp A, King E, Varigos G, Bahlo M, Forrest S (2001) The −590C/T and −34C/T interleukin-4 promoter polymorphisms are not associated with atopic eczema in childhood. J Allergy Clin Immunol 108:285–287
Enokihara H, Furusawa S, Nakakubo H, Kajitani H, Nagashima S, Saito K, Shishido H, Hitoshi Y, Takatsu K, Noma T et al (1989) T cells from eosinophilic patients produce interleukin-5 with interleukin-2 stimulation. Blood 73:1809–1813
Fung MC, Hapel AJ, Ymer S, Cohen DR, Johnson RM, Campbell HD, Young IG (1984) Molecular cloning of cDNA for murine interleukin-3. Nature 307:233–237
Gleich GJ (1990) The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol 85:422–436
Hang LW, Hsia TC, Chen WC, Chen HY, Tsai JJ, Tsai FJ (2003) Interleukin-10 gene -627 allele variants, not interleukin-I beta gene and receptor antagonist gene polymorphisms, are associated with atopic bronchial asthma. J Clin Lab Anal 17:168–173
Hedrick PW (1987) Gametic disequilibrium measures: proceed with caution. Genetics 117:331–341
Hedrick P, Kumar S (2001) Mutation and linkage disequilibrium in human mtDNA. Eur J Hum Genet 9:969–972
Hijazi Z, Haider MZ (2000) Interleukin-4 gene promoter polymorphism [C590T] and asthma in Kuwaiti Arabs. Int Arch Allergy Immunol 122:190–194
Hobbs K, Negri J, Klinnert M, Rosenwasser LJ, Borish L (1998) Interleukin-10 and transforming growth factor-beta promoter polymorphisms in allergies and asthma. Am J Respir Crit Care Med 158:1958–1962
Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY (1998) IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol 160:3555–3561
Karjalainen J, Hulkkonen J, Pessi T, Huhtala H, Nieminen MM, Aromaa A, Klaukka T, Hurme M (2002a) The IL1A genotype associates with atopy in nonasthmatic adults. J Allergy Clin Immunol 110:429–434
Karjalainen J, Nieminen MM, Aromaa A, Klaukka T, Hurme M (2002b) The IL-1beta genotype carries asthma susceptibility only in men. J Allergy Clin Immunol 109:514–516
Karjalainen J, Hulkkonen J, Nieminen MM, Huhtala H, Aromaa A, Klaukka T, Hurme M (2003a) Interleukin-10 gene promoter region polymorphism is associated with eosinophil count and circulating immunoglobulin E in adult asthma. Clin Exp Allergy 33:78–83
Karjalainen J, Joki-Erkkila VP, Hulkkonen J, Pessi T, Nieminen MM, Aromaa A, Klaukka T, Hurme M (2003b) The IL1A genotype is associated with nasal polyposis in asthmatic adults. Allergy 58:393–396
Kim YK, Cho SH, Koh YY, Son JW, Jee YK, Lee MH, Min KU, Kim YY (1999) Skin reactivity to inhalant allergens, total serum IgE levels, and bronchial responsiveness to methacholine are increased in parents of nonatopic asthmatic children. J Allergy Clin Immunol 104:311–316
Koh YY, Jeong JH, Kim CK, Kim YK, Jee YK, Cho SH, Min KU, Kim YY (2000) Atopic status and level of bronchial responsiveness in parents of children with acute bronchiolitis. J Asthma 37:709–717
Nakamura Y, Ozaki T, Kamei T, Kawaji K, Banno K, Miki S, Fujisawa K, Yasuoka S, Ogura T (1993) Increased granulocyte/macrophage colony-stimulating factor production by mononuclear cells from peripheral blood of patients with bronchial asthma. Am Rev Respir Dis 147:87–91
Noguchi E, Shibasaki M, Arinami T, Takeda K, Yokouchi Y, Kawashima T, Yanagi H, Matsui A, Hamaguchi H (1998) Association of asthma and the interleukin-4 promoter gene in Japanese. Clin Exp Allergy 28:449–453
Noguchi E, Nukaga-Nishio Y, Jian Z, Yokouchi Y, Kamioka M, Yamakawa-Kobayashi K, Hamaguchi H, Matsui A, Shibasaki M, Arinami T (2001) Haplotypes of the 5′ region of the IL-4 gene and SNPs in the intergene sequence between the IL-4 and IL-13 genes are associated with atopic asthma. Hum Immunol 62:1251–1257
Owen WF Jr, Rothenberg ME, Silberstein DS, Gasson JC, Stevens RL, Austen KF, Soberman RJ (1987) Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med 166:129–141
Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC (1995) Genetic susceptibility to asthma—bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med 333:894–900
Rand TH, Silberstein DS, Kornfeld H, Weller PF (1991) Human eosinophils express functional interleukin 2 receptors. J Clin Invest 88:825–832
Renauld JC (2001) New insights into the role of cytokines in asthma. J Clin Pathol 54:577–589
Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB (1992) Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326:298–304
Sandford AJ, Chagani T, Zhu S, Weir TD, Bai TR, Spinelli JJ, Fitzgerald JM, Behbehani NA, Tan WC, Pare PD (2000) Polymorphisms in the IL4, IL4RA, and FCERIB genes and asthma severity. J Allergy Clin Immunol 106:135–140
Shi HZ, Deng JM, Xu H, Nong ZX, Xiao CQ, Liu ZM, Qin SM, Jiang HX, Liu GN, Chen YQ (1998) Effect of inhaled interleukin-4 on airway hyperreactivity in asthmatics. Am J Respir Crit Care Med 157:1818–1821
Shute JK, Vrugt B, Lindley IJ, Holgate ST, Bron A, Aalbers R, Djukanovic R (1997) Free and complexed interleukin-8 in blood and bronchial mucosa in asthma. Am J Respir Crit Care Med 155:1877–1883
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
Takabayashi A, Ihara K, Sasaki Y, Suzuki Y, Nishima S, Izuhara K, Hamasaki N, Hara T (2000) Childhood atopic asthma: positive association with a polymorphism of IL-4 receptor alpha gene but not with that of IL-4 promoter or Fc epsilon receptor I beta gene. Exp Clin Immunogenet 17:63–70
Townley RG, Bewtra A, Wilson AF, Hopp RJ, Elston RC, Nair N, Watt GD (1986) Segregation analysis of bronchial response to methacholine inhalation challenge in families with and without asthma. J Allergy Clin Immunol 77:101–107
Walley AJ, Cookson WO (1996) Investigation of an interleukin-4 promoter polymorphism for associations with asthma and atopy. J Med Genet 33:689–692
Warringa RA, Koenderman L, Kok PT, Kreukniet J, Bruijnzeel PL (1991) Modulation and induction of eosinophil chemotaxis by granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood 77:2694–2700
Woolley KL, Adelroth E, Woolley MJ, Ramis I, Abrams JS, Jordana M, O’Byrne PM (1996) Interleukin-3 in bronchial biopsies from nonasthmatics and patients with mild and allergen-induced asthma. Am J Respir Crit Care Med 153:350–355
Yamada R, Tanaka T, Unoki M, Nagai T, Sawada T, Ohnishi Y, Tsunoda T, Yukioka M, Maeda A, Suzuki K, Tateishi H, Ochi T, Nakamura Y, Yamamoto K (2001) Association between a single-nucleotide polymorphism in the promoter of the human interleukin-3 gene and rheumatoid arthritis in Japanese patients, and maximum-likelihood estimation of combinatorial effect that two genetic loci have on susceptibility to the disease. Am J Hum Genet 68:674–685
Zhang J, Chen H, Hu L, Fu J, Zhang H, Chen Y (2002) Correlation between polymorphism of IL-4 and IL-10 gene promoter and childhood asthma and their impact upon cytokine expression. Zhonghua Yi Xue Za Zhi 82:114–118
Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA (1999) Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 103:779–788
Acknowledgements
This work was supported by grant number M1-0302-00-0073 of the National Research Lab. Program as part of National Research and Development Program from the Ministry of Science and Technology of Korea.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Park, B.L., Kim, L.H., Choi, Y.H. et al. Interleukin 3 (IL3) polymorphisms associated with decreased risk of asthma and atopy. J Hum Genet 49, 517–527 (2004). https://doi.org/10.1007/s10038-004-0184-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-004-0184-x
Keywords
This article is cited by
-
Interleukin-10 Gene Promoter Polymorphisms and Susceptibility to Asthma: Systematic Review and Meta-analysis
Biochemical Genetics (2021)
-
Interleukin-4 gene polymorphism (C33T) and the risk of the asthma: a meta-analysis based on 24 publications
BMC Medical Genetics (2020)
-
Interleukin 4 gene polymorphism (−589C/T) and the risk of asthma: a meta-analysis and met-regression based on 55 studies
BMC Immunology (2020)
-
Genetic variation in interleukin-7 is associated with a reduced erythropoietic response in Kenyan children infected with Plasmodium falciparum
BMC Medical Genetics (2019)
-
Impacts of different cytokine and chemokine polymorphisms in Pakistani asthmatics a case control study
COPD Research and Practice (2017)