Abstract
Two novel γ-butyrolactones ghanamycins A (1) and B (2) were isolated from the fermentation broth of marine-derived Streptomyces ghanaensis TXC6-16. Their structures were elucidated by spectroscopic analysis. These two novel compounds exhibited antimicrobial activities against some phytopathogens. The minimum IC (MIC) of 2 against Pseudomonas syringae and Erwinia sp. were 50 μg ml−1.
Similar content being viewed by others
Introduction
New marine nature products showing all the aspects of bioactivity have increased in recent years.1, 2 Marine actinomycetes, as an important group among marine microorganisms, are an abundant resource for unique biological activities because of their secondary metabolites with the structural diversity.3, 4 Butyrolactones are ubiquitous in microorganisms, especially in actinomycetes, and they are known as quorum-sensing signaling molecules for activating production of antibiotics.5 In recent years, many new γ-butyrolactones have been isolated from microorganisms.6, 7
To obtain antimicrobial secondary metabolites from marine-derived actinomycetes, we screened the antimicrobial substances produced by the marine Streptomyces sp. TXC6-16 isolated from Tamarix chinensis Lour. grown in the intertidal zone of the Yellow Sea, which lies in Shandong Province, China. In the preliminary experiments, the supernatant of the Streptomyces sp. TXC6-16 fermentation broth exhibited strong antimicrobial activity against some phytopathogens such as Fusarium oxysporum, Alternaria solani, Pyricularia oryzae, Collectotrichum lagenarium, Ralstonia solanacearum and Erwinia sp. In the course of our search for potential antimicrobial natural products from marine Streptomyces sp. TXC6-16 using bio-guided isolation, two novel γ-butyrolactones ghanamycins A (1) and B (2) were isolated. Their structures were elucidated by spectroscopic analysis. The MIC of 2 was only 50 μg ml−1 against Pseudomonas syringae and Erwinia sp. This paper reports the purification, structural determination and biological activity of these two novel γ-butyrolactones from Streptomyces sp. TXC6-16.
Results
Identification of the producing strain
The marine-derived Streptomyces sp. TXC6-16 was isolated from Tamarix chinensis Lour. grown in the intertidal zone of the Yellow Sea, which lies in Shandong Province, China. The strain was identified as Streptomyces ghanaensis according to its morphological characteristics, biochemical characteristics and partial sequence of its 16 S rDNA. The healthy strain was deposited at Marine Pharmaceutical Bank of First Institute of Oceanography, State Oceanic Administration (HTTA-F09018) and China General Microbiological Culture Collection Center (CGMCC10935).
Fermentation
The strain TXC6-16 was first cultured on Coates medium from agar slants for 3 days, then the healthy colonies were generated and inoculated into six 250 ml shake flasks which contained 50 ml of seed medium per flask. After cultured under 180 r.p.m. at 28 °C for 3 days, the seed medium was inoculated into 5 l fermenter that contained 3.0 l of fermentation medium with 10% inoculum concentration. After 7 days, the fermentation broth was centrifuged at 10 000 r.p.m. for 10 min to obtain the supernatant without sediment. The broth containing the target compounds was collected to 10 l in the above method.
Extraction and isolation
The supernatant was evaporated in vacuum and the crude extract was dissolved in acidification methanol (pH 1.7). The MeOH-extract was mixed with ethyl acetate (EtOAc; 1:6, v/v) and the precipitate was removed by centrifugation. The above method was conducted three times for collecting the EtOAc layer that was combined and concentrated in vacuum. The EtOAc extract (29.39 g) was obtained and subjected to silica gel column chromatography (CC) eluted with CHCl3/MeOH system (99:1, 95:5, 90:10, 80:20, 70:30, 60:40, 50:50 and 0:100, v/v). The active fraction of CHCl3/MeOH 9:1 (11.33 g) was then applied to Sephadex LH-20 column eluted with CHCl3/MeOH system (2:1, v/v) to obtain 45 fractions.
The active fractions 21–41 were combined (8.40 g) and further separated by ODS CC eluted with MeOH/H2O (60, 70, 80, 90 and 100%, v/v). The active fractions were obtained in 60 and 80% MeOH eluents.
The active fraction in 60% MeOH/H2O eluent was further purified by silica gel CC according to HPLC analysis. The eluent, which was eluted successively via stepwise gradient of CHCl3/MeOH (100:0–0:100, v/v), was used to obtain 8 fractions. The bioactive fraction of CHCl3/MeOH (95:5, v/v) was collected, combined and concentrated in a vacuum to yield a crude residue. This active fraction was subjected to purification via Sephadex LH-20 (Pharmacia, Uppsala and Sweden) eluted with CHCl3/MeOH (1:1, v/v) to obtain 5 active fractions. The fraction with the strongest activity was analyzed and prepared by HPLC (Agilent 1100, cosmoil C18, 5 μm, 20 × 250 mm, 18 ml min−1, double UV detection 210 nm/240 nm, tR=43 min) eluted with 12% CH3CN to yield compound 1 (15 mg).
The active fraction eluted with 80% MeOH/H2O was purified and prepared by Prep-TLC (CHCl3/MeOH 50:1, v/v) and HPLC (Waters 1525/2487, cosmoil C18, 5 μm, 20 × 250 mm, 7 ml min−1, double UV detection 210 nm/254 nm, tR=21 min) eluted with 50% CH3CN to yield compound 2 (12 mg).
Structural elucidation of compounds 1 and 2
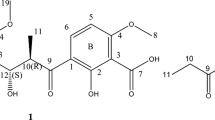
Compound 1 was obtained as a white powder by multi-step chromatography procedure from the fermentation broth of Streptomyces sp TXC6-16. It gave an [M+H]+ peak in the high-resolution electrospray-ionization MS (HR-ESI-MS) at m/z 317.0838 (calcd for C13H17O9, 317.0828), indicating the presence of 6 degrees of unsaturation. In the IR absorption spectrum, the absorption peak in 3440.2 showed the presence of the hydroxyl group. Intense absorptions between 1780 and 1740 cm−1 in the IR spectrum suggested the presence of a lactone bond and ester bond, respectively. The 1H and 13C NMR spectra (in CD3OD) showed 15 proton and 13 carbon signals (Table 1) that are classified into three methoxyl groups (δH 3.78/δC 54.0, 3.73/δC 53.2 and 3.65/δC 52.7), three methanes (δC 30.4, 30.1 and 28.9) and seven quaternary carbons, namely, three ester carbonyls (δC 174.7, 171.2 and 170.3), one lactone group (δC 169.4), one olefinic double bond (δC143.9 and 124.7) and one quaternary carbon C4 (δC 86.9). Classification was conducted through an analysis of heteronuclear multiple quantum correlation and DEPT spectra. The chemical shift in the quaternary carbon C4 is δC 86.9, indicating that C4 was hydroxylated. No olefinic protons were observed in the 1H NMR spectrum (Table 1), thereby suggesting that the C=C double bond was fully substituted. The olefinic double bond (δC 143.9, 124.7) indicated that the double bond was exocyclic, which differed from that of 2, 3-disubstituted γ-hydrobutenolide compounds.8 Compound 1 is a unique γ-butyrolactone derivative. The molecular structure of compound 1 was confirmed by the HMBC correlations between H-5 and C3/C4/C7, as well as between H-6 and C3/C7. Therefore, the planar structure of 1 was assigned (Figure 1).
Compound 2 was obtained as light yellow crystal. It gave an [M+H]+ peak in the HR-ESI-MS data at m/z 401.1819 (calcd for C18H29O9, 401.1767). The 1H and 13C NMR spectra (in CDCl3) for 2 (Table 2) were very similar to that of 1. Therefore, compounds 1 and 2 were analogs (Figure 2). The marked difference was that C9 and C10 of compounds 1 and 2 connected to different substituent groups. C9 and C10 of compound 1 connected with the methoxyl group, whereas C9, C10 of compound 2 connected with the butoxyl group. The molecular structure of compound 2 was confirmed by the HMBC correlations between H-2 and C3/C4/C8; H-5 and C3/C4; H-1′ and C7; and H-1′′ and C9/C3′′. The planar structure of 2 (Figure 3) was elucidated to be the same as that of 1 by similar analysis of spectroscopic data.
Ghanamycin A ( 1): white powder; UV (MeOH) λmax (logɛ): 240 nm; [α]25D=0 (c0.05, CH3OH). HRESIMS m/z 317.0838 [M+H]+ (calcd for C13H17O9, 317.0828); 1H and 13C NMR data see Table 1.
Ghanamycin B ( 2): light yellow crystal; UV (MeOH) λmax (logɛ): 254 nm; [α]25D=0 (c0.05, CH3OH). HRESIMS m/z 401.1819 [M+H]+ (calcd for C18H29O9, 401.1767); 1H and 13C NMR data see Table 2.
Biological properties
The antimicrobial activities shown in Table 3 indicated both 1 and 2 exhibited broad spectrum activities against phytopathogens such as F. oxysporum, Colletotrichum sp., Erwinia sp. and P. syringae.
Compounds 1 and 2 were evaluated for their antimicrobial activities against the tested plant pathogenic microorganisms. The MIC of 2 against certain phytopathogenic bacteria, such as P. syringae and Erwinia sp., was only 50 μg ml−1. However, compound 1 showed weak inhibitory activity against the tested plant pathogens.
Discussion
Two novel γ-butyrolactones ghanamycins A (1) and B (2) were isolated from marine-derived actinomycetes. The key structural features of compounds 1 and 2 were characterized by spectroscopic analyses. Antimicrobial activities were evaluated using MIC assays. Compound 1 exhibited weak activities against both phytopathogenic bacteria and fungi, but compound 2 exhibited antimicrobial activities against certain phytopathogenic bacteria, such as P. syringae and Erwinia sp., with only 50 μg ml−1, and moderate antimicrobial activities. The strain TXC6-16 was identified as S. ghanaensis according to its morphological characteristics, biochemical characteristics and partial sequence of its 16 S rDNA.
In many Streptomyces species, antibiotic production and morphological differentiation are controlled by small diffusible signaling molecules.9 γ-Butyrolactone, as a signal molecule, combines with its receptor for regulating morphological differentiation and production of antibiotics in streptomycetes.10 The most intensively studied γ-butyrolactones is A-factor (2-isocapryloyl-3 R-hydroxymethyl-γ-butyrolactone), which is required for streptomycin production and sporulation in Streptomyces griseus.10 Other γ-butyrolactones have also been shown to induce antibiotic biosynthesis, such as the virginiae butanolides of Streptomyces virginiae.11, 12 At least 60% of Streptomyces species produce γ-butyrolactones,12 and these compounds are likely to play important roles as extracellular signaling molecules in the biology of such organisms. Further research is necessary to determine whether these two novel γ-butyrolactones ghanamycin A and B are signaling molecules for inducing antibiotic production and morphological differentiation.
Methods
General
Fractions were monitored with TLC (HSGF 254, Yantai, China), and spots were visualized by heating silica gel plates sprayed with 5% H2SO4 in 95% ethanol. CC was performed on Sephadex LH-20 (Pharmacia) and silica gel (200–300 mesh, Yantai, China). HPLC purification was conducted on Waters 1525/2487 and Agilent 1100 liquid chromatography. UV spectra were performed on a Shimadzu–UV–1700 spectrophotometer. IR spectra were performed on a Magna–IR 550 spectrometer. NMR experiments were performed on Bruker AVANCE-500 and AVANCE-400 instruments. HR-ESI-MS spectrum was acquired using a Q-Tof micro LCTTM mass spectrometer. EI-MS was performed on a Q-Tof micro instrument (XEVO G2 TOF Mass Analyzer, waters, USA) at a capillary voltage of 3 kV, sample cone voltage of 80 V, an extraction cone voltage of 4 V, source temperature of 80 °C, desolvation temperature of 150 °C, ion energy of 1 V, MCP detector of 2200 V and collision energy of 10 V (MS).
Antimicrobial activity
The antimicrobial activities of 1 and 2 were determined by the serial twofold agar dilution methods13 using potato dextrose agar media for plant pathogens such as F. oxysporum after incubation for 48 h at 30 °C. The tested strains were cultivated on LB medium for bacteria at 37 °C and on potato dextrose agar (PDA) medium for fungi at 28 °C.
The tested compounds were dissolved in MeOH at different concentrations from 1500 μg ml−1to 50 μg ml−1 by the continuous twofold dilution methods. The MICs were defined as the lowest concentration at which no visible growth of microbes could be observed.
References
Blunt, J. W., Copp, B. R., Munro, M. H. G., Northcote, P. T. & Prinsep, M. R. Marine natural products. Nat. Prod. Rep. 27, 165–237 (2010).
Mayer, A. M. S., Rodríguez, A. D., Berlinck, R. G. S. & Berlinck, M. T. Hamann: marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 1790, 283–308 (2009).
Zotchev, S. B. Marine actinomycetes as an emerging resource for the drug development pipelines. J. Biotechnol. 158, 168–175 (2012).
Ramesh, S. & William, A. Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol. Res. 167, 571–580 (2012).
Du, Y. L., Shen, X. L., Yu, P., Bai, L. Q. & Li, Y. Q. Gamma-butyrolactone regulatory system of Streptomyces chattanoogensis links nutrient utilization, metabolism, and development. Appl. Environ. Microbiol. 77, 8415–8426 (2011).
Raju, R., Garcia, R. & Müller, R. Angiolactone, a new butyrolactone isolated from the terrestrial myxobacterium, Angiococcus sp. J. Antibiot. 67, 725–726 (2014).
Zhang, X. M., Zhang, D. F., Li, W. J. & Lu, C. H. Pseudonocardides A–G, new γ-butyrolactones from marine-derived Pseudonocardia sp. YIM M13669. Helv. Chim. Acta 99, 191–196 (2016).
Arakawa, K., Tsuda, N., Taniguchi, A. & Kinashi, H. The butenolide signaling molecules SRB1 and SRB2 induce lankacidin and lankamycin production in Streptomyces rochei. Chembiochem 13, 1447–1457 (2012).
Khokhlov, A. S. et al. The A-factor, responsible for streptomycin biosynthesis by mutant strains of Actinomyces streptomycini. Dokl. Akad. Nauk SSSR 177, 232–235 (1967).
Takano, E. et al. Purification and structural determination of SCB1, a γ-butyrolactone that elicits antibiotic production in Streptomyces coelicolorA3(2). J. Biol. Chem. 275, 11010–11016 (2000).
Horinouchi, S. & Beppu, T. Autoregulatorsin. Genetics And Biochemistry Of Antibiotic Production 103–119 Butterworth-Heinemann, Newton, MA, USA, (1994).
Yamada, Y., Nihira, T. & Sakuda, S. in Biotechnology of Antibiotics 2nd edn, (ed. Strohl W. R.) 63–79 (CRC Press, Boca Raton, USA, 1997).
Jorgensen, J. H. & Turnidge, J. D. in Manual of Clinical Microbiology, 8th edn Vol. 9, 1108–1127 ASM Press, Washington, DC, USA, (2003).
Acknowledgements
This work was financially supported by the High-Tech Program (863 Program) of China (2008AA09Z407), National Natural Science Foundation of China No. 40776098 and 40976104, and the National Special Fund for State Key Laboratory of Bioreactor Engineering, Grant No 2060204.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
All authors contributed equally to this work and declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, Jh., Gu, Kb., Zhang, DJ. et al. Ghanamycins A and B, two novel γ-butyrolactones from marine-derived streptomyces ghanaensis TXC6-16. J Antibiot 70, 733–736 (2017). https://doi.org/10.1038/ja.2017.37
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.37