Abstract
Currently, the quality-control strategy of amphotericin B in US Pharmacopoeia, British Pharmacopoeia, European Pharmacopoeia and Pharmacopoeia of the People’s Republic of China all adopt the combination of purity measurement by HPLC and potency measurement by microbiological assay. In this study, we prepared pure amphotericin B and quantified the relationship between amphotericin B content and potency values using the mass-balance method and microbiological assay. The potency of amphotericin B with an absolute purity of 100% was determined to be 1048.63 U mg–1. An HPLC method was then established to simultaneously determine the content and potency of amphotericin B, which unified the quality-control procedure for amphotericin B. A good linear relationship was observed between the peak area and the concentration, which could be expressed as y=113074x+4196.5, R2=0.9999. The lower limit of quantification was 0.4473 ng. The HPLC method is expected to become the routine quality-control method and replace the current laborious quality-control procedure in pharmacopoeias.
Similar content being viewed by others
Introduction
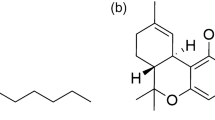
Amphotericin B is a polyene antifungal antibiotic produced by Streptomyces nodosus. Its molecular formula is C47H73NO17, and its chemical structure is shown in Figure 1. Amphotericin B changes the permeability of cell membrane by combining with ergosterol on the fungal cell membrane to result in intracellular sodium and potassium ion leakage, thus killing the fungal cells.1, 2, 3 Amphotericin B is the preferred drug for deep fungal infection. However, its adverse effects, such as serious renal toxicity, have restricted its clinical application. In recent years, new formulations such as liposomal amphotericin B have been developed that have significantly reduced toxicity and side effects, and amphotericin B has regained wide clinical application.4, 5, 6 Although amphotericin B has been used in clinical practice since the 1960s, there was limited research progress on its quality control. To date, the pharmacopoeias of various countries still use microbial potency to characterize its content.7, 8, 9, 10
The characterization of antibiotics content by potency has been practiced since the early days of antibiotics development, and is currently still in use. Nowadays, the units of antibiotics potency and purity have largely been unified during drug development. Therefore, for antibiotics with unambiguous structure and single component, chemical analysis has generally substituted the traditional microbiological assay in pharmacopoeias to determine antibiotics content. Because of its unique characteristics such as specificity, the HPLC method has been recommended for determination of antibiotics potency since the late 1980s,11, 12, 13 and is gradually adopted in the pharmacopoeias of various countries. However, for some antibiotics developed earlier, often their potency cannot be directly determined by HPLC because the relationship between their potency and purity is not clearly quantified. For such antibiotics, the product quality is usually ensured by the combined control of HPLC purity and microbiological potency measurement. Currently, all major pharmacopoeias use this protocol for the quality control of amphotericin B.7, 8, 9, 10 Amphotericin B is manufactured by fermentation, which may produce biologically active analog impurities that can affect the accuracy of the microbiological potency assay result. Although the analysis of amphotericin B by HPLC has been previously reported,14, 15 the quantitative relationship between potency and purity of amphotericin B remained unclear. In our earlier work,16 we prepared pure vancomycin and norvancomycin, and determined the quantitative relationship between potency and purity values using microbiological assay and HPLC, and thus established an HPLC method to determine the potency of the main active ingredient of vancomycin and norvancomycin. The 2005 edition of Chinese Pharmacopoeia replaced the traditional microbiological assay with the HPLC method to determine the content of norvancomycin.7 In this paper, we elucidate the quantitative relationship between potency and purity of amphotericin B using the same strategy. The unified measurement of microbiological potency and purity of amphotericin B is shown in Figure 2. Consequently, we improved the HPLC method reported in the literature and established an HPLC method for the simultaneous determination of the potency and the purity of amphotericin B.
Results and discussion
Improvement of HPLC analysis of amphotericin B
The HPLC analysis of amphotericin B reported in the literature used either a weak acid or the chelating agent EDTA in the mobile phase. The weak acid improves the chromatographic peak shape by inhibiting the chelation of amphotericin B with metal ions in the chromatographic system under acidic condition, and EDTA improves the chromatographic behavior of amphotericin B by direct competition against amphotericin B for chelation with metal ions.17 The organic phase in the mobile phase included methanol and acetonitrile. Our study found that tetrahydrofuran can significantly enhance the separation of amphotericin B from the impurities. The optimized organic phase in the mobile phase contained 41/18/10 methanol/acetonitrile/tetrahydrofuran, and the mobile phase comprised 55/45 organic phase/buffer (2.5 mmol l–1 EDTA-2Na).
The HPLC methods reported in the literature often detect amphotericin B and heptaene impurities at 407 nm.18, 19 It can be seen from the full-wavelength scan spectrum of amphotericin B that heptaene compounds show a maximum absorption peak at 383 and 407 nm (Figure 3). Using 383 nm as the detection wavelength will not affect the detection sensitivity and can also avoid repeated switching of the deuterium and tungsten lamps of the detector during the analysis. Therefore, in this study, 383 nm was selected to identify amphotericin B and heptaene impurities and 303 nm was selected to identify tetraene impurities according to the reference.20
HPLC methodology validation
The improved HPLC method was validated with the International Conference on Harmonization guidelines.21
Specificity
The known impurities in amphotericin B include heptaene impurities; that is, impurity B (amphotericin X1),10 amphotericin B (5)22 and the hexaene impurities; that is, impurity A (amphotericin A)10 and nystatin10 (Figure 4). We injected the European Pharmacopoeia impurity reference solution and the impurity mixture solution, respectively, and found that in our improved HPLC method the known impurities can be completely separated (Figure 4).
Linearity
Ten sample solutions at different concentrations (0.2–201 μg ml–1) were prepared from a suitable quantity of amphotericin B reference standard tested to give the slope intercept of the regression equation: y=113074x+4196.5, R2=0.9999. The results showed a good linear relationship between the amphotericin B peak area, concentration in the range of 0.2–201 μg ml–1.
Precision
Amphotericin B reference solutions at three concentrations (0.5448, 0.7264 and 0.9080 μg ml–1) were prepared and injected. Three replicate runs were conducted for each concentration, and determinations were performed for three consecutive days. The average relative standard deviation of intra-day repeatability was 0.146%, and the average relative standard deviation of inter-day repeatability was 0.531%.
Lower limit of detection and limit of quantification
The lower limit of detection (S/N=3) and the lower limit of quantification (S/N=10) of amphotericin B were 0.1342 and 0.4473 ng, respectively.
Solution stability
The relative standard deviation (n=12) of the area of the amphotericin B principal peak was 0.011% for the reference solution prepared within 12 h, stored at 4 °C, and protected from light.
Quantitative relationship between purity and potency of amphotericin B
The HPLC purity of the prepared pure amphotericin B was 99.66% and the relative standard deviation was 0.003% (n=6). The amphotericin B assay was determined to be 95.53% by the mass-balance method (Table 1). The potency of the pure amphotericin B was determined by the three-dose method and the single-dose method of microbiological assay. By combined calculation, the potency of the pure amphotericin B was found to be 1001.76 U mg–1, with the range of confidence limit being 968.39–1035.13 U mg–1 (Table 1). The potency of the pure amphotericin B was calculated to be 1043.93 U mg–1 on the dried basis. Therefore, the potency of amphotericin B with an absolute purity of 100% was determined to be 1048.63 U mg–1, which was used as a constant in equation (1) to calculate the potency based on the assay of amphotericin B by HPLC.
Unified determination of amphotericin B content and potency
The potency of amphotericin B can be calculated on the basis of amphotericin B content from the HPLC assay using equation (1):

where At is the peak area of the test sample, As is the peak area of the reference standard, Vt is the dilution volume of the test sample, Vs is the dilution volume of the reference standard, Wt is the weight of the test sample, Ws is the weight of the reference standard, Ps is the amphotericin B content of the reference standard and 1048.63 is a constant. Thus, an HPLC method is available for the simultaneous determination of amphotericin B content and potency.
Three batches of amphotericin B drug were used to validate the above method. All three batches had >90% HPLC purity. The potency values of amphotericin B calculated from equation (1) were slightly lower than the values determined by microbiological assay (Table 2). As impurities in amphotericin B have some biological activity,2, 22 lower sample purity will inevitably result in larger systematic error between the value determined by microbiological assay and the calculated value obtained by HPLC.
Methods
Instruments, samples and reagents
Pure amphotericin B was prepared by preparative HPLC at our laboratory, using a Shimadzu HPLC system (Shimadzu, Tokyo, Japan; LC-20AT Separations Module, SPD-M20A photodiode array detector, LC solution chemstation, preparative HPLC column: Shim-pack PREP-ODS). The peak purity of pure amphotericin B by UV is shown in Figure 5. The peak purity index of pure amphotericin B is 0.999999. US Pharmacopoeia amphotericin B reference standard (A1960629 US Pharmacopoeia, potency 1103 μg mg–1 on dried basis) and European Pharmacopoeia amphotericin B impurity reference standard (code: Y0001014, Batch No.: 1.0) were used. Three batches of amphotericin B drug samples (Batch No.: A1960695, A1960696, A1960697) manufactured by Axellia Pharmaceuticals (Oslo, Norway) were used to validate the developed HPLC method. Chromatographic-grade methanol, acetonitrile and tetrahydrofuran manufactured by Fisher (Pittsburgh, PA, USA) were used in the experiment.
Preparation of pure amphotericin B
Crude amphotericin B was dissolved in DMF (5.0 mg ml–1) solution. The preparation and separation of pure amphotericin B were then carried out on a PREP-ODS C18 column using the following mobile phase: 15:85 water–organic phase (43/20 methanol/acetonitrile). The flow rate was 8.0 ml min–1 and the injection volume was 1.0 ml. Detection wavelength was set to 383 and 303 nm. The fractions of the principal peak were collected and combined, concentrated on a rotary evaporator to remove the organic solvents, then purified by Sephadex-LH20 gel again using water as the mobile phase, and then freeze-dried to give pure amphotericin B.
HPLC method
Column: Phenomenex luna C18 (250 × 4.6 mm, 5 μm); mobile phase: 55/45 organic phase (41/18/10 methanol/acetonitrile/tetrahydrofuran)–buffer (2.5 mmol l–1 EDTA-2Na); flow rate: 1.0 ml min–1; column temperature: 30 °C; detection wavelength: 383, 303 nm; injection volume: 20 μl.
Microbiological assay
The potency was determined by the titer plate microbiological assay method, as described in the monograph of amphotericin B in Chinese Pharmacopoeia.7
Assay of pure amphotericin B by the mass-balance method
The formula for calculation of the assay percentage by the mass-balance method23, 24 is as follows in equation 2

In this experiment, the impurity was determined with reference to the HPLC principal component. The volatile impurities were determined by weight loss on drying.7 The residue on ignition was determined by the conventional method.7
Solution preparation
European Pharmacopoeia impurity reference solution
European Pharmacopoeia impurity reference standard (0.0010 g) was weighed and dissolved in 10 ml DMF to obtain a solution containing amphotericin B, impurity B and impurity A.
Acid degradation impurity solution
Amphotericin B (0.0055 g) was weighed and transferred to a 50-ml volumetric flask, dissolved in 3 ml DMF and diluted with methanol to 50 ml. The pH of the above-mentioned solution was adjusted to about 2 with 0.1 mol l–1 hydrochloric acid. The solution was allowed to stand at room temperature for 4 h to produce the acid degradation product impurity B (amphotericin X1).2
Amphotericin B (5) solution
Amphotericin B (0.0972 g) was weighed and transferred to a 10-ml volumetric flask, dissolved in DMF and diluted with DMF to 10 ml. The solution was then subjected to accelerated degradation in a water bath at 60 °C for 50 h to produce amphotericin B (5).22
Impurity mixture solution
Equal volumes of the above-mentioned acid degradation impurity solution and the amphotericin B (5) solution were mixed. A known amount of nystatin reference standard was then added to the solution, dissolved and thoroughly mixed.
Conclusion
This study determined the quantitative relationship between the amphotericin B content and potency values using the mass-balance method and microbiological assay. An HPLC method for the simultaneous determination of amphotericin B content and potency was established and validated. This HPLC method can be an alternative to the traditional microbiological assay for the determination of amphotericin B, as it eliminates the need to use both HPLC and microbiological assay in the quality-control protocol for amphotericin B. The method has the potential to become the routine quality-control method for amphotericin B in pharmacopoeias.
References
Gallis, H., Drew, R. & Pickard, W. Amphotericin B: 30 year of clinical experience. Clin. Infect. Dis. 12, 308–329 (1990).
Li, J., Zhu, H. Q., Li, J. Y., Jin, S. H. & Hu, C. Q. Isolation, structure elucidation and activity of an unknown impurity of amphotericin B. J. Antibiot. 60, 272–276 (2007).
Tran-Dinh, S. et al. Effects of amphotericin B on the glucose metabolism in Saccharomyces cerevisiae cells. studies by 13C, 1H-NMR and biochemical methods. Eur. J. Biochem. 197, 271–279 (1991).
Minodier, P., Robert, S. & Noel, G. First-line liposomal amphotericin B for pediatric visceral leishmaniasis in southern France. Arch. Pediatr. 12, 1102–1108 (2005).
Adedayo, A. et al. A pharmacokinetic study of amphotericin B lipid complex injection (Abelcet) in patients with definite or probable systemic fungal infections. Antimicrob. Agents Chemother. 44, 2900–2902 (2000).
Martino, R. et al. No signs of new toxicity when amphotericin B lipid complex (ABLC, Abelcet) and caspofungin are used in combination in the treatment of FUO. Blood (Ash. Annu. Meet. Abstr.) 104, 5077 (2004).
The China Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China (in Chinese) 2005 edn (China Chemical Industry Press, Beijing, 2005).
The United States Pharmacopoeia Commission Inc. The United States Pharmacopeia NF23 (The United States Pharmacopeia Convention, Inc, Rockville, MD, 2005).
The British Pharmacopoeia Commission. The British Pharmacopoeia (The Stationery Office, London, 2003).
The European Pharmacopoeia Commission. The European Pharmacopoeia 6th edn (Council of Europe, Strasbourg, 2007).
Thomas, A. H. Replacement of microbiological assay by high-performance liquid chromatographic assay for antibiotics. J. Pharm. Biomed. Anal. 5, 319–324 (1987).
Vanderhaeghe, H. Replacement of microbiological assay of antibiotics based on high-performance liquid chromatography. J. Pharm. Biomed. Anal. 7, 127–128 (1989).
Hoogmartens, J. Liquid chromatography for the quantitatives analysis of antibiotics—some applications using poly(styene-divinylbenzene). J. Pharm. Biomed. Anal. 10, 845–850 (1992).
Margosis, M. & Aszalos, A. Quantitation of amphotericins by reverse-phase high-performance liquid chromatography. J. Pharmac. Sci. 73, 835–838 (1984).
Zhu, H. Q., Hu, C. Q. & Zhao, X. RP-HPLC determination of amphotericin B and its liposome. Chin. J. Pharm. Anal. 26, 949–952 (2006).
Liu, M. & Hu, C. Q. Simultaneous determination of purity and potency of vancomycin and norvancomycin by high-performance liquid chromatography. Chromatographia 65, 203–207 (2007).
Zhu, W. C. & Wang, L. B. Determination of content of amphotericin B of amphotericin B liposomal for injection by RP-HPLC. Pharm. Care Res. 6, 382–383 (2006).
Yan, X. Y., Li, Z. M., Wang, A. J., Ren, B. B. & Wang, Y. W Content determination of amphotericin B and its related substances by HPLC. Chin. J. Antibiot. 31, 551–554 (2006).
Xie, Q. F., Tang, X. L., Song, X. Z. & Zhao, Z. X. Determination of amphotericin B in human plasma by reversed-phase HPLC. Chin. J. Mod. Appl. Pharm. 18, 126–128 (2001).
Ma, J. W., Liu, Y. B., Wen, D. X., Zhu, F. J. & Wang, S. W. Studies on the composition of nystatin by high performance liquid chromatography. Acta Pharm. Sin. 20, 294–300 (1985).
ICH. Q3A(R): Impurities in New Drug Substances (The ICH Steering Committee, Switzerland, 2002).
Wang, Y. H., Zhang, J. P., Chang, Y. & Hu, C. Q. A newly identified derivative of amphotericin B: isolation, structure determination and primary evaluation of the activity and toxicity. J. Antibiot. 63, 553–557 (2010).
The Directorate for the Quality of Medicines of the Council of Europe in European Pharmacopoeia 5th edn, Supplement 5.6, General texts 5.12.4 (Council of Europe, Strasbourg, 2007).
World Health Organization in International Pharmacopoeia 3rd edn, Vol. 5, Supplementary information, Part A, 3.3 (World Health Organization, Geneva, 2003).
Acknowledgements
Project supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (Grant No. 2010ZX09401-403).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, Y., Wang, YH. & Hu, CQ. Simultaneous determination of purity and potency of amphotericin B by HPLC. J Antibiot 64, 735–739 (2011). https://doi.org/10.1038/ja.2011.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2011.83
Keywords
This article is cited by
-
Consistency evaluation between matrix components ratio and microbiological potency of tylosin major components
DARU Journal of Pharmaceutical Sciences (2018)