Abstract
Platensimycin and platencin are inhibitors of FabF and FabF/H bacterial fatty acid synthesis enzymes, respectively. Discovery of natural congeners provides one of the ways to understand the relationship of chemical structure and biological function. Efforts to discover the natural analogs of platensimycin by chemical screening led to the isolation of platensimycin B4, a glucoside congener of platensimycin. This analog showed significantly attenuated activity and critically defined the limited binding space around the aromatic ring and established the importance of the free phenolic and carboxyl group for the activity.
Similar content being viewed by others
Introduction
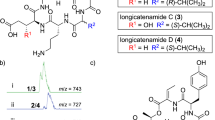
The discovery of platensimycin (1) and platencin (2) (Figure 1) from various strains of Streptomyces platensis generated significant excitement within the antibiotic research community.1, 2, 3, 4 Discovery of these compounds was possible due to the design and introduction of a novel antisense differential sensitivity screening strategy in which FabH/FabF was sensitized.3, 4, 5, 6 Platensimycin is a selective inhibitor of the FabF acyl-enzyme intermediate and platencin is a balanced dual inhibitor of both FabH and FabF. Both of these compounds showed potent in vitro activities in both cell-free and whole-cell assay systems. The in vitro activities translated well in an animal model when dosed with intravenous continuous infusion. However, lack of in vivo activity was observed under conventional administration due to poor systemic exposure. To improve PK properties and in vivo efficacy various approaches were undertaken, including chemical modification of platensimycin at some of the chemically accessible sites.7, 8 Discovery of congeners is an alternative and complementary approach, which was also simultaneously undertaken to augment structure activity relationship studies. This approach has allowed us the discovery of a number of congeners including platensimide A,9 homoplatensimide A,10 platensimycin B1–B3,11 platensimycin A112 and platencin A1.13
Continued searching for additional congeners and related analogs from a large-scale fermentation of S. platensis by chemical screening led to the isolation of a new platensimycin glucoside (3a) and its methyl ester (3b) (Figure 1). This compound provided critical information about the scope of substitutions in the aromatic ring and their relationship to the antibacterial activity. The isolation, structure elucidation and biological activity of 3a and 3b are herein described.
Results and discussion
Streptomyces platensis MA7327 was fermented in 70 l stirred tanks for 9 days on CLA production medium (consisting of g/l, yellow corn meal, 40, lactose, 40, ambrerex pH 5, antifoaming agent (P2000), 0.8 ml/l; 3% inoculum) and was extracted with MeOH at pH 2.7. The extract was chromatographed on reversed phase Amberchrome resin and eluted with a step gradient of aqueous MeOH. The late-eluting fractions were basified to pH 9, extracted with CH2Cl2 and chromatographed on a reversed phase C8 HPLC to afford 3a (9.2 mg, 0.21 mg/l) and 3b (7.2 mg, 0.17 mg/l) both as amorphous powders.
Platensimycin B4 (3a) produced parent ions at m/z 626 [M+Na] and m/z 602 [M-H] in positive and negative ion modes, respectively, suggesting a molecular weight of 603. High resolution electrospray ionization Fourier transformation mass spectrometry analysis showed a protonated molecular ion at m/z 604.2372 (M+H) and afforded a molecular formula of C30H37NO12, which differed from platensimycin by C6H10O5. The formula was supported by the 13C NMR spectrum (Table 1), which showed the presence of 30 resonances and looked similar to platensimycin with the presence of six additional all oxygenated resonances. The IR spectrum showed the presence of the absorption bands for hydroxy group, conjugated ketone, carboxylic acid and aromatic systems. The UV spectrum showed three absorption bands (λmax 217, 245 and 295 nm), which differed from platensimycin, indicating minor changes in the aromatic moiety. The electrospray ionization MS spectrum showed a fragment ion at m/z 273, characteristic of the fragment ion for platensic acid moiety due to the cleavage of the amide bond (Figure 2). The 1H and 13C NMR spectra of 3a were identical to the corresponding spectra of platensimycin, except for the presence of additional resonances corresponding to six additional carbons and associated protons. The presence of intact platensimycin was confirmed by COSY, total correlation spectroscopy, heteronuclear multiple quantum coherence and heteronuclear multiple bond coherence (HMBC) correlations. The five of the six addition carbons present in the 13C NMR spectrum resonated in between δC 62.3 and 78.0 p.p.m., and the sixth carbon resonated at δC 102.6 p.p.m. The last carbon correlated with a doublet (J=7.4 Hz) appearing at δH 4.98 (H-1′′), indicative of an anomeric carbon. The double quantum-filtered COSY and total correlation spectroscopy correlations confirmed the presence of a hexose moiety, which was elucidated to a glucoside by HSQC and HMBC correlations. The anomeric proton showed HMBC correlation to C-5′′, thus confirming a pyranoside. All protons of the sugar moiety showed large couplings suggesting that all substitutions of the hexose moiety were equatorial, thus confirming the presence of glucoside. The anomeric proton δH 4.98 showed an HMBC correlation to the oxygenated aromatic carbon C-5′ (δC 157.1), thus establishing the glycosidic linkage at C-5′. This was further supported by the NOE enhancements of H-6′ on irradiation of the anomeric proton (δH 4.98), which also showed NOEs to H-3′′ and H-5′′, confirming the β-D-glucoside configuration. These NOEs were also confirmed in the methyl ester (3b). In addition, irradiation of H-6′ of 3b exhibited NOE to H-1′′ further corroborating the glycosidic linkage at C-5′. The mass spectrum of methyl ester 3b showed a molecular weight of 617, which was up by a 14 A.M.U. (CH2). The 1H and 13C NMR spectrum (Table 1) of 3b was identical to 3a, except for the presence of a methoxy signal assigned for the methyl ester δC 52.9 (δH 3.92, s). The methoxy singlet (δH 3.92) displayed an HMBC correlation to the C-1′ carbonyl, thus confirming the methyl ester. On the basis of these data structure 3a, a platensimycin-5′-β-D-glucoside was assigned to platensimycin B4.
Biological activity
Platensimycin B4 (3a) and the methyl ester (3b) were evaluated in various in vitro cell-free and cell-based assays and the results are summarized below. In the Staphylococcus aureus cell-free FASII assay,14 which measures the inhibitory activity of compounds against fatty acid synthesis, 3a and 3b showed IC50 values of 160 and 40 μg/ml (320- and 80-fold less active than platensimycin), respectively. Similar results were also obtained against saFabH assay, in which 3a and 3b showed IC50 values of 100 and 35 μg/ml, respectively. The two- to three-fold better activity exhibited by the methyl ester was somewhat surprising and is in odds with platensimycin and its methyl ester. Compounds 3a and 3b each showed very poor antibacterial activity minimum detection concentration (defined by minimum concentration of the compound showing differential zone of clearance between antisense plates compared with control plate)5 of 1000 μg ml−1 in the most sensitive (antisense two-plate differential sensitivity) assay, which is 25 000-fold worse than platensimycin.1 As these compounds showed poor activity in all of the assays described above, it was not surprising that they did not show any antibacterial activity against wild-type S. aureus and many other bacterial strains at less than 64 μg ml−1. In cell-free FASII assay, 3b and 3a were only 80- and 320-fold less active than platensimycin; however, their antibacterial activity against most sensitive antisense assay was 25 000 poorer than that of platensimycin. This suggested that 5′-glucosylation not only decreases enzyme inhibition activity but may also have blocked the entry to bacterial cells.15, 16 The loss of activity of platensimycin B4 could be easily attributed to the loss of water-mediated hydrogen bond of C-5′ hydroxy group, as observed in the platensimycin bound X-ray crystal structure (Figure 3).
In summary, we have described the isolation, structure elucidation and biological characterization of platensimycin B4 and its methyl ester. Platensimycin B4, a 5′-glucosyl derivative of platensimycin, lost all of the antibacterial activity and has shown significant attenuation of cell-free activity suggesting that the bulky polar groups at C-5′ are not tolerated for the biological activities.
Methods
Optical rotations were measured with a Perkin-Elmer 241 polarimeter (Perkin-Elmer, Waltham, MA, USA). UV spectra were recorded on a Beckman DU-70 spectrophotometer (Beckman, Rockville, MD, USA). IR spectra were recorded with a Perkin-Elmer Spectrum One FT-IR spectrophotometer. All NMR spectra were recorded with a Varian Unity 500 (1H, 500 MHz, 13C, 125 MHz) spectrometer (Varian, Palo Alto, CA, USA). The residual protons of solvents were used as internal reference (DMSO-d6 δH 2.49, δC 39.5; C5D5N δH 8.74, δC 150.35). The COSY, double quantum-filtered COSY, DEPT, total correlation spectroscopy, heteronuclear multiple quantum coherence and HMBC spectra were measured using standard Varian pulse sequences. Electrospray ionization MS data were recorded on an Agilent 1100 MSD (Agilent, Santa Clara, CA, USA) with ESI ionization. High resolution electrospray ionization Fourier transformation mass spectrometry was acquired on a Thermo Finnigan LTQ-FT (Thermo Fisher Scientific, Waltham, MA, USA) with the standard Ion Max API source (without the sweep cone) and ESI probe. Three scan events were used. The ion trap was scanned from 150 to 2000 first in negative ion mode and then in positive ion mode. The FT was scanned from 200 to 2000 in the positive ion mode only. In all cases, the SID was set to 18 V to try to reduce multiple ion clusters. Instrument resolution is set to 100,000 at m/z 400. No internal calibration was required; the instrument was calibrated once a week and checked daily to assure accuracy. An Agilent HP1100 instrument was used for analytical HPLC.
Fermentation condition
A 1 ml frozen suspension of S. platensis MA7327 (ATCC PTA-5316) was inoculated in 50 ml ATCC-2 seed medium (2% inoculum) in a 250 ml baffled Erlenmeyer flask and incubated at 28 °C at 220 r.p.m. on a shaker for 48 h. The second stage seed was developed by aseptic transfer of 8 ml broth from the stage-1 seed into three 2 l non-baffled Erlenmeyer flasks, containing 500 ml of ATCC-2 medium (consisting of in g per l, starch, 20, dextrose, 10, NZ amine type E, 5, beef extract, 3, peptone, 5, yeast extract, 5, calcium carbonate, 1) each (1.6% inoculum), and cultivated for 48 h at 180 r.p.m. shaker speed at 28 °C. Pooled contents from all three flasks (1500 ml) were transferred into 50 l of ‘CLA’ production medium in a 70 l fermentation tank. The production batch (43 l volume) was harvested after 9 days at 5 psig pressure, 300 r.p.m. agitation, 7 slpm airflow at 28 °C.
Isolation of platensimycin B4 (3a) and methyl ester (3b)
The fermentation broth (43 l) was diluted with 29 l methanol and shaken for 60 h at room temperature. The mixture was then centrifuged in 1 l bottles at 5000 r.p.m. for 45 min. The supernatant was diluted to 40% methanol; the pH was adjusted to 2.7 with 2N HCl and concentrated to a volume of 11 l using a 500 kDa ultrafiltration membrane. The filtrate (11 l, aqueous-MeOH ratio 60:40) was charged directly on to a 1.6 l Amberchrome column. The column was eluted with a step gradient of 40–100% aqueous methanol with 10% increments of MeOH. Two 600 ml fractions were collected from each 10% MeOH increments affording 14 fractions (Amberchrome fractions 1–14). Amberchrome fractions 10–12 eluting with 80–90% MeOH were pooled and concentrated under reduced pressure, to remove most of the MeOH, to a volume of 200 ml mostly containing water. Water (300 ml) was added to this extract to produce a final volume of 500 ml, which was basified to pH ∼9 by addition of solid sodium bicarbonate. The basic solution was extracted three times with methylene chloride (3 × 450 ml). The methylene chloride extract was dried over anhydrous Na2SO4 and filtered. Solid Na2SO4 was washed with small amount of MeOH. MeOH and CH2Cl2 filtrates were concentrated separately under reduced pressure to give 0.314 g (MeOH fraction) and 1.77 g (CH2Cl2 fraction), respectively. The MeOH fraction was chromatographed by reversed-phase HPLC (Zorbax C8, 21.2 × 250 mm, 40 min gradient of 20–80% aqueous CH3CN, each containing 0.05% TFA at 10 ml/min). Fraction eluting at 13 min was lyophilized to yield 9.2 mg (0.21 mg/l) of platensimycin B4 (3a) as an amorphous powder.
The CH2Cl2 fraction (1.77 g) was chromatographed on a 1 l Sephadex LH-20 (GE Health Care Bio-Sciences AB, Uppsala, Sweden) column and eluted with a step gradient of one column volume each of hexane/CH2Cl2 (1:4), acetone:CH2Cl2 (1:4) and acetone/CH2Cl2 (3:2) at a flow rate 10 ml/min. The column was unpacked and LH20 was washed with MeOH. All fractions were analyzed by silica gel TLC and reversed phase HPLC before pooling. The methanol wash fraction was concentrated to give 224.5 mg residue, which was chromatographed on reversed-phase HPLC (Zorbax C8, 21.2 × 250 mm, eluted with 25% aqueous CH3CN with 0.05% TFA for 40 min followed by a 40 min gradient of 25–100% aqueous CH3CN at 10 ml/min). Fractions eluting at 37–40 min were pooled and extracted with EtOAc. EtOAc extract was concentrated to furnish 7.2 mg (0.17 mg/l) of the methyl ester 3b as an amorphous powder. 3a: [α]23D -28 (c, 0.5, MeOH), UV (MeOH) λmax 217 (ɛ 14 665), 245 (8040), 295 (2815) nm, FTIR (ZnSe) 3284, 2929, 1676 (br, strong), 1440, 1277, 1206, 1138 cm−1 electrospray ionization MS (m/z) 626 [M+Na], 442 [M-glu+H], 273; 602 [M-H]; high resolution electrospray ionization Fourier transformation mass spectrometry (m/z) 604.2372 (calcd for C30H37NO12+H: 604.2316), for 1H and 13C NMR see Table 1. 3b: [α]23D -56 (c 0.5, MeOH), UV (MeOH) λmax 221 (ɛ 24 830), 250 (13 611), 301 (4405) nm, FTIR (ZnSe) 3320, 2958, 1672(br, strong), 1503, 1440, 1337, 1279, 1203, 1074 cm−1, electrospray ionization MS (m/z) 640 [M+Na], 600, 456, 273; 730 [M+TFA-H]; 616 [M–H]; high resolution electrospray ionization Fourier transformation mass spectrometry (m/z) 618.2531 (calcd for C31H39NO12+H: 618.2550), for 1H and 13C NMR see Table 1.
FabF2P Assay
Staphylococcus aureus cells (RN450) carrying plasmid S1–1941 bearing antisense to FabF (fabF AS-RNA strain) or vector (control strain) were inoculated from a frozen vial source into a tube containing 3 ml of Miller's LB Broth (Invitrogen, Carlsbad, CA, USA) plus 34 μg/ml of chloramphenicol. Tubes were incubated at 37 °C at 220 r.p.m. for 18–20 h and kept at room temperature until use. Miller's LB broth was supplemented with 1.2% Select agar (Invitrogen), 0.2% glucose, 15 μg/ml chloramphenicol and 50 mM of xylose (for the antisense strain only). The OD600 (3.0) of the culture was measured and diluted by aforementioned agar media to 1:1000 (OD600 0.003). Aliquots (100 ml) of agar were poured into each 20 × 20 cm NUNC plates, and well-caster templates placed into the agar and the agar was allowed to solidify for 30 min. Then, 20 μl of test samples were added to the wells and the plates incubated at 37 °C for 18 h and zones of inhibition measured.5 Minimum detection concentration values were determined by twofold serial dilution.
FASII Assay
The assay was performed in a phospholipid-coated 96-well flash plate. Briefly, 1.26 μg of the partially purified protein from S. aureus containing all the required fatty acid synthesis enzymes was preincubated with a serial dilution of natural products at room temperature for 20 min in 50 μl of buffer containing 100 mM sodium phosphate (pH 7.0), 5 mM EDTA, 1 mM NADPH, 1 mM NADH, 150 μM DTT, 5 mM β-mercaptoethanol, 20 μM n-octanoyl-CoA (or lauroyl-CoA), 4% Me2SO and 5 μM ACP. The reaction was initiated by addition of 10 μl of water-diluted [2-14C]-malonyl-CoA, giving a final concentration of 4 μM malonyl-CoA with total counts of about 10 000 c.p.m. The reaction was incubated at 37 °C for 90 min. The reaction was terminated by adding 100 μl of 14% perchloric acid. The plates were sealed and incubated at room temperature overnight and counted for 5 min using Packard TopCount NXT scintillation counter (Packard, Meriden, CT, USA). Further details of the assay are described in the study by Kodali et al.14
Antibiotic assay (MIC)
The MIC against each of the strains was determined by twofold dilution as previously described5, 14 and conducted under National Committee for Clinical Laboratory Standards (NCCLS)—now called the Clinical Laboratory Standards Institute—guidelines.5, 14 Cells were inoculated at 105 CFU/ml, followed by incubation at 37 °C with a twofold serial dilution of compounds in the growth medium for 20 h. MIC is defined as the lowest concentration of antibiotic inhibiting visible growth.
References
Wang, J. et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature. 441, 358–361 (2006).
Singh, S. B. et al. Isolation, structure, and absolute stereochemistry of platensimycin, a broad spectrum antibiotic discovered using an antisense differential sensitivity strategy. J. Am. Soc. 128, 11916–11920. and 15547 (2006).
Jayasuriya, H. et al. Isolation and structure of platencin: a novel FabH and FabF dual inhibitor with potent broad spectrum antibiotic activity produced by Streptomyces platensis MA7339. Angew. Chem. Int. Ed. 46, 4684–4688 (2007).
Wang, J. et al. Platencin is a dual FabF and FabH inhibitor with potent in vivo antibiotic properties. Proc. Natl Acad. Sci. (USA) 104, 7612–7616 (2007).
Young, K. et al. Discovery of FabH/FabF inhibitors from natural products. Antimicrob. Agents. Chemother. 50, 519–526 (2006).
Singh, S. B., Phillips, J. W. & Wang, J. Highly sensitive target based whole cell antibacterial discovery strategy by antisense RNA silencing. Curr. Opin. Drug. Disc. Dev. 10, 160–166 (2007).
Singh, S. B., Herath, K. B., Wang, J., Tsou, N. N. & Ball, R. G. Chemistry of platensimycin. Tetrahedron. Lett. 48, 5429–5433 (2007).
Shen, H. C. et al. Synthesis and biological evaluation of platensimycin analogs. Bioorg. Med. Chem. Lett. 19, 1623–1627 (2009).
Herath, K. B. et al. Structure and semisynthesis of platensimide A, produced by Streptomyces platensis. Org. Lett. 10, 1699–1702 (2008).
Jayasuriya, H. et al. Structure of homoplatensimide A: a potential key biosynthetic intermediate of platensimycin isolated from Streptomyces platensis. Tetrahedron. Lett. 49, 3648–3651 (2008).
Zhang, C. et al. Isolation, structure and fatty acid synthesis inhibitory activities of platensimycin B1-B3 from Streptomyces platensis. Chem. Commun. (Camb) 5034–5036 (2008).
Singh, S. B. et al. Isolation, enzyme-bound structure and antibacterial activity of platensimycin a1 from Streptomyces platensis. Tetrahedron. Lett. 50, 5182–5185 (2009).
Singh, S. B. et al. Isolation, enzyme-bound structure and antibacterial activity of platencin A1 from Streptomyces platensis. Bioorg. Med. Chem. Lett. 19, 4756–4759 (2009).
Kodali, S. et al. Determination of selectivity and efficacy of fatty acid synthesis inhibitors. J. Biol. Chem. 280, 1669–1677 (2005).
Young, K. & Silver, L. L. Leakage of periplasmic enzymes from enva1 strains of escherichia coli. J. Bacteriol. 173, 3609–3614 (1991).
Markham, P. N. & Neyfakh, A. A. Efflux-mediated drug resistance in Gram-positive bacteria. Curr. Opin. Microbiol. 4, 509–514 (2001).
Acknowledgements
We thank S Kodali, A Galgoci, K Young, R Painter, M Motyl and K Dorso for some of the initial biological results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Ondeyka, J., Guan, Z. et al. Isolation, structure and biological activities of platensimycin B4 from Streptomyces platensis. J Antibiot 62, 699–702 (2009). https://doi.org/10.1038/ja.2009.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2009.106
Keywords
This article is cited by
-
Synthesis and Antifungal Activity of Indole Derivatives
Chemistry of Natural Compounds (2023)
-
Novonestmycins A and B, two new 32-membered bioactive macrolides from Streptomyces phytohabitans HBERC-20821
The Journal of Antibiotics (2015)
-
Isolation and structural elucidation of glucoside congeners of platencin from Streptomyces platensis SB12600
The Journal of Antibiotics (2013)