Abstract
The symbiotic association of corals and unicellular algae of the genus Symbiodinium in the southern Persian/Arabian Gulf (PAG) display an exceptional heat tolerance, enduring summer peak temperatures of up to 36 °C. As yet, it is not clear whether this resilience is related to the presence of specific symbiont types that are exclusively found in this region. Therefore, we used molecular markers to identify the symbiotic algae of three Porites species along >1000 km of coastline in the PAG and the Gulf of Oman and found that a recently described species, Symbiodinium thermophilum, is integral to coral survival in the southern PAG, the world’s hottest sea. Despite the geographic isolation of the PAG, we discovered that representatives of the S. thermophilum group can also be found in the adjacent Gulf of Oman providing a potential source of thermotolerant symbionts that might facilitate the adaptation of Indian Ocean populations to the higher water temperatures expected for the future. However, corals from the PAG associated with S. thermophilum show strong local adaptation not only to high temperatures but also to the exceptionally high salinity of their habitat. We show that their superior heat tolerance can be lost when these corals are exposed to reduced salinity levels common for oceanic environments elsewhere. Consequently, the salinity prevailing in most reefs outside the PAG might represent a distribution barrier for extreme temperature-tolerant coral/Symbiodinium associations from the PAG.
Similar content being viewed by others
Introduction
Tropical coral reefs represent a major natural resource that depends on the habitat-forming scleractinian coral species and their symbiosis with dinoflagellate algae of the genus Symbiodinium (zooxanthellae). Damage to the algal symbiont can result in the breakdown of this association leading to often fatal coral bleaching. Episodes of mass bleaching events that have contributed to global reef degradation have been linked to high-temperature anomalies. The threshold temperature for heat-induced bleaching typically lies only 1 °C above the average maximum summer temperature for a given region (Goreau and Hayes, 1994; Baker et al., 2008) and will be reached more frequently as sea surface temperatures rise. The resulting increased frequency of mass bleaching has been predicted to devastate coral reef populations on a global scale within decades (Baker et al., 2008; van Hooidonk et al., 2013; Logan et al., 2014).
Furthermore, environmental factors such as reduced salinity and changes in the concentrations of dissolved inorganic nutrients can promote bleaching (Goreau, 1964; Coles, 1992; Wiedenmann et al., 2013; D’Angelo and Wiedenmann, 2014). Coral reef survival will strongly depend on the adaptive and acclimatory responses of corals and their symbionts to the pressure of global warming (Logan et al., 2014). The understanding of these adaptive processes requires the knowledge of the distribution of species with a high-temperature stress tolerance.
The highest known bleaching thresholds are found in coral communities in the Persian/Arabian Gulf (PAG), which represent a biogeographic subset of Indo-Pacific coral communities (Coles, 2003; Sheppard et al., 1992). Despite their exceptional capacity to survive higher temperature as compared to corals from elsewhere, PAG corals remain susceptible to bleaching when temperatures exceed their local thresholds. Recently, major bleaching events have resulted in a strong decline of PAG coral communities (Burt et al., 2013; Coles and Riegl, 2013; Riegl et al., 2011; Sheppard and Loughland, 2002). Nevertheless, while conspecifics or close relatives of PAG corals usually bleach and die at temperatures ⩾32 °C elsewhere (Coles and Riegl, 2013), coral symbioses in the PAG endure summer peak temperatures of up to 36 °C (Coles and Riegl, 2013; Hume et al., 2013). Notably, they also need to withstand unusually low temperatures which can drop below 20 °C during winter (Coles, 1992; Coles, 2003; Sheppard et al., 1992).
Symbiodinium clade D, certain members of which are considered to increase the heat tolerance of its coral hosts, has been found in the northwestern (off Saudi Arabia) and northeastern (off Iran) PAG (Baker et al., 2004; Ghavam Mostafavi et al., 2007; LaJeunesse et al., 2014), prompting the hypothesis that this symbiont might be responsible for the heat tolerance in this region. We found, however, that Symbiodinium of the internal transcribed spacer 2 (ITS2)-type (‘subclade’) C3 is the year-round prevalent symbiont in corals of Saadiyat reef in the southern PAG where clade D is essentially absent (Hume et al., 2013; Hume et al., 2015). This was surprising, since members of subclade C3 are generally considered to be ‘cosmopolitan, thermally sensitive generalists’ (Lajeunesse, 2005). Further investigation revealed that the representatives of this ‘subclade’ from the PAG are in fact a separate species, Symbiodinium thermophilum (Hume et al., 2015). The genetically distinct nature of this species became obvious from the analysis of the non-coding region of the chloroplast psbA gene (psbAncr) and was confirmed by the specific sequences of domain V of the chloroplast large subunit ribosomal DNA (cp23S) and the mitochondrial cytochrome b gene (cob; Hume et al., 2015). Furthermore, we have described an ITS2 variant (termed C3-Gulf ITS2 variant), which contains a characteristic 8-bp sequence duplication and occurs at low abundance among S. thermophilum populations (Hume et al., 2015). These results are in agreement with previous findings, which demonstrate that subclade C3 consists of different species lineages that cannot be resolved by molecular phylogenetic analysis using solely the ITS2 region of the nuclear ribosomal DNA sequences (LaJeunesse and Thornhill, 2011; Thornhill et al., 2014).
The ability to distinguish S. thermophilum from other subclade C3 symbionts enabled us to assess for the first time whether this heat-tolerant symbiont is widely distributed in the southern PAG and can also occur outside this waterbody. This is of particular interest given the geographic isolation of the PAG from the adjacent Gulf of Oman and the wider Indian Ocean through the only 42-km-wide Strait of Hormuz. Because of the restricted water exchange, shallow depths (mean 30 m), high evaporation rates and limited freshwater input, the PAG waters are characterised by high salinities (often 42–44). The resulting halocline circulation is sustained by a net inflow of surface water into the PAG and limited outflow of heavier, more saline bottom waters (Sheppard et al., 1992; Johns et al., 1999; Yao and Johns, 2010). Consequently, PAG corals and their algal symbionts have to cope with the highest salinity levels reported globally for coral reef ecosystems (Coles, 2003; Feary et al., 2013). Furthermore, the net inward flow of seawater likely inhibits the movement of coral larvae and other pelagic organisms from the PAG into adjacent seas (Sheppard et al., 1992).
To assess whether S. thermophilum is widely distributed in the southern PAG and whether it can also occur outside the PAG, we focused our study on Porites lobata, P. lutea and P. harrisoni since all individuals of these corals analysed so far in the Southern PAG hosted this symbiont species as the year-round dominant partner (Hume et al., 2015). Moreover, the genus Porites represents an important framework builder in most reefs of the world including the PAG (Burt et al., 2008; Loya et al., 2001) and P. lobata hosting S. thermophilum is a well-characterised laboratory model for physiological experiments with PAG corals (Hume et al., 2013).
Materials and methods
Sample collection and processing
Samples of P. lobata, P. lutea and P. harrisoni colonies were collected at different sites within the PAG, the Strait of Hormuz and the Gulf of Oman in September 2012 and March 2013. Samples were removed from the top of the colonies from a depth range of 2–7 m and transferred to the surface in individual containers for immediate fixation in ethanol absolute or freezing in dry ice. For best results, genomic DNA was extracted using a previously described cetyltrimethylammonium bromide-based protocol (Hume et al., 2013). DNA was dissolved in deionized water and stored frozen at −20 °C. For molecular taxonomy purposes, the ITS1-5.8 S-ITS2 region of the rDNA operon of zooxanthellae was amplified using the primers SYM-VAR_FWD and SYM_VAR_REV and PCR conditions as already published (Hume et al., 2013). These primers were previously optimised to avoid contaminations of the amplicon with Porites host sequences. The PCR products were purified with the Jetquick Gel Extraction Spin Kit (GenoMed, Leesburg, FL, USA) and stored frozen for downstream analyses.
Determination of zooxanthellae phylotypes
To determine the phylotypes of zooxanthellae by sequencing, the PCR-amplified ITS1-5.8 S-ITS2 region of the rDNA operon was cloned using the StrataClone PCR cloning kit (Agilent, Santa Barbara, CA, USA) and plasmid DNA was prepared from Escherichia coli colonies using a Fermentas GeneJET plasmid Miniprep kit (Thermo Scientific, Waltham, MA, USA). Sequencing services were provided by Macrogen Inc (Seoul, South Korea) and Eurofins Genomics (Ebersberg, Germany) and the 220-bp sequence of the ITS2 region was subjected to BLAST searches (www.ncbi.nlm.nih.gov/blast). Subsequently, the ITS2 phylotype of the identical/most similar reference sequences available in GenBank was assigned to the query sequence. Representative ITS2 sequences including those of (sub)clades C3, C3-Gulf ITS2 variant, D and C15 cluster from every sampled region were submitted to GenBank (accession numbers: KJ563083-KJ563104). Clade information for Montipora foliosa was retrieved from Wiedenmann et al. (2013). The 55 coral colonies examined in this study returned 519 zooxanthellae ITS2 sequences. Sequences of 25 colonies from Dalma, Saadiyat and Umm Al Quwain previously used in Hume et al. (2015) to define S. thermophilum were included for comparison.
To determine the dominant zooxanthellae (sub)clade present in Porites colonies, the ITS2 region was analysed by PCR and denaturing gradient gel electrophoresis (DGGE) as detailed in Supplementary Methods. Briefly, the ITS2 region was amplified by using genomic DNA as template in a PCR reaction with the primer pair SYM_VAR_5.8SII and SYM_VAR_Clamp. DGGE analysis was conducted using a Bio-Rad DCode system (Bio-RAD, Hercules, CA, USA) for DGGE with a model 475 gradient former. The taxonomic information contained in characteristic gel patterns was established by subjecting DNA extracted from representative bands to further analyses including sequencing. Thereby, DNA markers of all Symbiodinium (sub)clades relevant to this study were generated and subsequently processed along with the samples. Two representative DGGE gels are shown in Supplementary Figure S1. The DGGE phylotypes were determined for 305 coral colonies. The dominant phylotypes of 22 colonies from Dalma, Saadiyat and Umm Al Quwain from Hume et al. (2015) were included for comparison.
psbAncr amplification and molecular phylogeny
The non-coding region of the chloroplast psbA gene (psbAncr) was amplified from zooxanthellae of 109 P. lobata, P. lutea and P. harrisoni coral colonies collected at sites along ~1000 km of UAE and Oman coastline from September 2012 to March 2013. Out of 109 coral colonies analysed for this study, 22 were previously phylotyped (Hume et al., 2015) and included for comparison.
The psbAncr was amplified from frozen DNA using the primers psbAFor_1 and psbARev_1 with thermal cycling as described in LaJeunesse and Thornhill (2011). PCR reaction conditions were as described for the SYM_VAR_FWD and SYM_VAR_REV primer pair in Hume et al. (2013).
Amplicons were directly sequenced using the internal primer psbA_int_Fwd, 5′-CTAGGTATGGAAGTGATGCATG-3’; sequencing services were provided by Eurofins Genomics. Sequence chromatograms were checked by eye for potential sequencing errors. Any sequence with a chromatogram characteristic of multiple PCR products (for example, where several peaks were registered for a single nucleotide position or where reading frame shifts were apparent) was discarded from further analysis (~7.5% of all directly sequenced PCR amplicons).
The phylogenetic resolution of psbAncr sequences of ITS2-type C3 zooxanthellae associated with Porites spp. from the PAG and the Gulf of Oman (127 sequences) was assessed in relation to psbAncr sequences of ITS2-type C3 zooxanthellae and closely related variants from corals elsewhere retrieved from databases (266 sequences). Sequences were first aligned using ClustalW in MEGA6 (Molecular Evolutionary Genetics Analysis software, http://www.megasoftware.net/mega.php), checked by eye and edited manually. Phylogenies were estimated through Bayesian inference using MrBayes 3.2.24 (MrBayes:Bayesian Inference of Phylogeny software, http://mrbayes.sourceforge.net/index.php) applying the Jukes-Cantor model with a gamma-shaped distribution with invariable sites. The MCMC (Markov Chain Monte Carlo) analyses were run for 7.0 × 106 generations, sampling every 500 generations. A relative burn-in of 0.25 was used in calculating a 50% majority rule consensus tree. Nodal support was estimated using posterior probabilities (PPs). A non-Gulf C3-type psbAncr sequence collected in the Great Barrier Reef was used as an outgroup (accession number JQ043643). Pairwise genetic distances between psbAncr haplotypes were calculated with MEGA6 using all substitutions and with gaps considered via pairwise deletion.
Coral culture and physiological experiments
Replicate colonies (Ø ~3.5 cm) of two previously characterised laboratory strains of PAG P. lobata originating from Saadiyat reef, UAE (Hume et al., 2013) associated with S. thermophilum and of Indo-Pacific representatives of M. foliosa, Montipora sp. and P. lichen were co-cultured in compartments of our experimental mesocosm at the National Oceanography Centre, Southampton at a constant temperature of 27.5 °C and a 12 h/12 h light/dark cycle at salinity levels of 42 and 36.5 (Practical Salinity Scale 1978, PSS-78). The key element of this approach is that the replicate colonies of the PAG and Indo-Pacific species were not assessed in isolation, but kept/treated always in parallel in the same experimental compartments. In this setting, the corals exposed to their native salinity did not only serve as controls for the salinity treatment, but also at the same time as ‘environmental controls’ for the overall conditions of the experimental system.
Both compartments were fully equipped to sustain stable coral growth (D'Angelo and Wiedenmann, 2012; Hume et al., 2013). To keep the environmental conditions comparable, three times per week, 30% of the water was exchanged between the two systems. During the procedure, water from the high-salinity system (~0.87 litre per litre of the intended exchange volume) was topped up with reverse osmosis water (~0.13 litre per litre of the intended exchange volume) to reach the required salinity of 36.5. The water from the reduced salinity system could be directly incorporated into the high-salinity system, also to compensate for evaporation losses. The regular mixing of the two waterbodies kept water parameters other than salinity comparable.
When corals were to be studied at a salinity level different from that of their origin, they were first acclimated for 4 weeks to the altered salinity. To analyse heat-stress responses, the temperature of flow-through compartments integrated in the high and reduced salinity systems were ramped up by 0.5 °C per day and kept constant at 32 °C. The heat treatment tanks constantly circulated water from the control tanks, which was heated up when entering the experimental tank, thereby exposing the corals to the same water chemistry as in the respective controls. The physiological performance of the corals was assessed in two independent 5-month experiments, one measuring the colony weight, the other the live tissue cover, the heat-stress treatment were carried out in parallel. Growth or decay of the corals was quantified either by determining the weight after a 30-s drip-off period on absorbent tissue or by calculating the area of the corals covered with live tissue using ImageJ (http://rsbweb.nih.gov/ij/) analysis of digital photographs. Micrographs were produced with a Leica MZ10 stereo microscope (Leica Microsystems, Wetzlar, Germany).
Data analyses
The different strains/species of model corals were each represented by five replicate colonies in the treatments and in the controls of experiments carried out at ambient temperature. Four replicate colonies of each strain/species were used in the treatment and control of the heat-stress experiments to keep the number of killed animals small. Data of coral growth determined by changes in weight and live tissue cover were normalised to the initial values recorded after the acclimation period. The normalised data were fitted to theoretical models as defined in Supplementary Table S1. Two-sample t-tests were performed to evaluate the significance of the difference between the values obtained in each experiment at different salinity treatments. All analyses were performed using Origin 8.6.0 software (OriginLab Corporation, Northampton, MA, USA).
Results
Biogeographic distribution of extreme temperature-tolerant coral–zooxanthellae associations
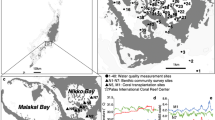
The answer to the question whether the heat tolerance of PAG corals is related to the presence of specific symbiont types is an integral part of the understanding of the exceptional resilience of corals in this region. Therefore, we identified the dominant dinoflagellate symbionts by analysing the rDNA ITS2 region (Supplementary Methods) of three Porites species (P. lobata, P. lutea and P. harrisoni) sampled along >1000 km of coastline of the southern PAG and the Gulf of Oman (Figure 1). In the PAG (highest temperatures and high-salinity water), 100% of these corals (>130 independent colonies from five locations) host Symbiodinium C3-type zooxanthellae (Figure 1a; Supplementary Figure S2 and Supplementary Table S2). Our analysis of a large set of symbiont data revealed that this specific partnership has not been found in coral reefs elsewhere in the world where P. lobata/P. lutea host almost exclusively C15-type Symbiodinium (217 analysed colonies, Figure 1b; Supplementary Figure S2 and Supplementary Table S3). We also detected representatives of this unusual C3-type association in the Gulf of Oman (high temperatures and normal oceanic salinity water), but its abundance decreased dramatically with the distance to the connecting Strait of Hormuz (Figure 1; Supplementary Figure S2). At the border zone of high-/normal-salinity water bodies (Musandam), the percentage of C3-associated Porites spp. dropped to 43%. With increasing distance to Musandam/Strait of Hormuz, the percentage reduced further to 18% (Fujairah) and to 4% in Muscat. At this latter location, 75% of the colonies were associated with C15-type zooxanthellae, the P. lobata/P. lutea symbiont usually found elsewhere (Figure 1; Supplementary Figure S2 and Supplementary Table S3). Symbiodinium clade D was detected in some Porites individuals collected in the transition zone between the PAG and the Gulf of Oman.
Global distribution of Porites spp.–Symbiodinium associations. (a) Schematic depiction of the sampling sites and oceanographic conditions in the PAG and the western Gulf of Oman. Seasonally averaged (July to September) surface salinity fields (coloured contour plot) and currents (black arrows) are overlaid using the 10-year model output (climatological run) from Yao and Johns (2010). The major surface inflow and deep outflow currents that connect the PAG with the Gulf of Oman are denoted by the thick arrows coloured according to their prevailing salinity. A minor coastal surface outflow (Johns et al., 1999) is represented by a solid white arrow. The sampling sites of the present study in the southern PAG, Musandam and the Gulf of Oman are highlighted by coloured filled circles. The percentage of Porites spp. (P. lobata, P. lutea and P. harrisoni) colonies that harbour C3 (determined by DGGE; Supplementary Table S2) in the different regions is colour coded in the figure and defined in the legend. The total number of analysed colonies is indicated in brackets (n), sample sizes of the individual locations are detailed in Supplementary Figure S2. Literature data for reefs elsewhere are specified in Supplementary Table S3. Salinity and current imagery was kindly provided by W.E. Johns, University of Miami. (b) Global distribution of P. lobata/P. lutea associations with Symbiodinium cladal/subcladal types (as defined by the internal transcribed spacer 2 (ITS2) region of the Symbiodinium rDNA operon). Pie chart size and filled colours represent the number of individual coral colony associations identified and ITS2 cladal/subcladal type, respectively. Integers within the pie charts refer to the sampling regions (Supplementary Table S3). Data for the PAG (G) and Musandam/Gulf of Oman (M/GO) from the present study are included for comparison (Supplementary Table S2).
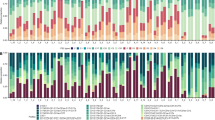
About 23% of the >510 ITS2 sequences of Porites-associated Symbiodinium subclade C3 contained the C3-Gulf ITS2 variant (sensu (Hume et al., 2015)) characteristic for S. thermophilum (Figure 2a; Supplementary Table S4). This sequence was found in every sampling location in at least ~40% of the analysed Porites colonies with C3-type zooxanthellae and also in C3-associated Porites colonies in the Gulf of Oman.
Geographic distribution of Porites/Symbiodinium associations. (a) Proportion of Symbiodinium rDNA ITS2-type C3 and C3-Gulf ITS2 variant marker sequences detected in Porites spp. corals from within eight locations in the PAG and the Gulf of Oman (Dalma to Muscat, Figure 1a; Supplementary Table S4) and in corals from reference regions. All available reference C3 sequences were retrieved from GeoSymbio (https://sites.google.com/site/geosymbio/database) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and included independent of the taxonomic background of the host corals. The number of analysed sequences/number of analysed colonies are shown in brackets next to the bars. (b) Phylogenetic tree produced by Bayesian inference analysis of psbAncr sequences from C3-type zooxanthellae samples from the PAG, Musandam and the Gulf of Oman (colour coded as indicated in the legend) and from zooxanthellae of the C3-type and closely related variants from elsewhere (regions detailed in the figure). psbAncr sequences of S. thermophilum from the PAG (Hume et al., 2015) were included for reference (open circles). Branches are drawn to scale as indicated by the bar. The separation of the S. thermophilum group in the PAG and the Gulf of Oman from the group containing the available sequences from elsewhere in the world is supported with a posterior probability value of 1.0. All accession numbers of the psbAncr sequences used in the analysis are given in Supplementary Tables S5 and S6.
Phylogenetic analysis of psbAncr sequences by Bayesian inference was performed for C3-type zooxanthellae samples from the PAG and the Gulf of Oman as well as for those representing the non-Gulf C3-type and closely related variants from elsewhere. Inferred phylogenies resolved the PAG and Gulf of Oman sequences as a well-supported group (PP=1.0) genetically distinct from non-Gulf C3-type sequences, giving average between-group genetic distances of 0.299 (PAG vs non-Gulf) compared with average within-group genetic distances of 0.049 and 0.092 for Gulf ITS2-type C3 and non-Gulf C3 sequences, respectively. The S. thermophilum group contains distinct clusters, which may indicate that it comprises of several distinct genetic lineages.
Importantly, this analysis resolved all available psbAncr sequences from C3-type zooxanthellae from the Gulf of Oman (Fujairah and Muscat) and Strait of Hormuz (the tip of the Musandam peninsula) in the same grouping as S. thermophilum reference sequences and samples from inside the PAG acquired for the present study (Figure 2b; Supplementary Tables S5 and S6).
With increasing distance to the main distribution area in the southern PAG (100% prevalence), the abundance of Porites colonies hosting symbionts of the S. thermophilum group decreases steadily along the coast of the Gulf of Oman and from there on becomes essentially undetectable in other parts of the world.
Thermal tolerance of corals associated with S. thermophilum at reduced salinity
Corals have limited tolerance to changes in salinity (Coles, 1992). Since the reduced abundance of Porites spp. associated with symbionts of the S. thermophilum group in the Gulf of Oman is correlated with a remarkable drop in salinity levels, the question arises whether the prevalence of this association in the southern PAG is related to the exceptionally high-salinity levels of these waters. Therefore, we conducted experiments under controlled laboratory conditions to assess the heat tolerance of S. thermophilum-hosting PAG corals at lower-salinity levels found in reefs elsewhere. For this purpose, we cultured replicate colonies of two strains of P. lobata originating from Saadiyat reef in the southern PAG (Hume et al., 2013) in our experimental mesocosm (D'Angelo and Wiedenmann, 2012) at salinity levels commonly observed in PAG waters (high salinity, 42) and the adjacent Gulf of Oman (reduced salinity, 36.5) at a constant temperature of 27.5 °C. Both strains host S. thermophilum (Supplementary Table S4). Replicate colonies of three species of Indo-Pacific corals (M. foliosa/Symbiodinium clade C, Montipora sp./Symbiodinium subclade C21/C3u and P. lichen/Symbiodinium subclade C21.1/C96) that originated from reefs with normal oceanic salinity levels in the range of 31–34 (D'Angelo and Wiedenmann, 2012) were included as environmental controls in the study. Keeping corals adapted to different native salinity levels together in the respective experimental compartments allowed us to confirm that the regular water exchange between the high- and low-salinity systems sustained comparably good water quality in both set-ups.
We did not observe obvious changes in the appearance of PAG corals at salinity levels found in the Gulf of Oman during the first 3 weeks of the experiments in the absence of temperature stress (Figures 3a and d). However, prolonged monitoring of changes in colony weight (Figures 3a and b) and in live tissue area (Figures 3c and d) in two independent 5-month experiments showed that, eventually, growth of P. lobata/S. thermophilum from the PAG is strongly reduced (strain 1) or stops completely (strain 2) in the low-salinity treatment. In an independent experiment, we exposed PAG P. lobata/S. thermophilum acclimated to high and reduced salinity to elevated temperatures of 32 °C, which represents a common summer temperature in the Gulf of Oman as reported by (Coles, 2003). The experimental specimens cultured at lower salinity ceased to grow (strain 1; Figure 3e) or lost 90% of their live tissue after ~63 days and died eventually (strain 2; Figure 3f). In contrast, the corals cultured at 32 °C and high salinity continued to grow as indicated by the increase in live tissue area (Figures 3e and f), although at a slower rate compared to the controls kept at 27.5 °C (Figures 3c and d).
Effect of reduced salinity on the growth of P. lobata/S. thermophilum associations from the PAG. Growth of replicate colonies of two strains (designated 1 and 2, left and right panels, respectively) of PAG P. lobata/S. thermophilum is shown at high (42) or reduced (36.5) salinity levels. Changes in weight (a and b) and colony surface area (c and d) covered by live tissue were determined in independent experiments. The normalised data represent average values of the corresponding replicates in each experiment, bars denote s.e.m. Lines display theoretical model fittings performed as described in Supplementary Table S1. (e and f) Replicate colonies of PAG P. lobata/S. thermophilum (strains 1 and 2) were acclimated to 36.5 or 42 salinity levels at 27.5 °C. At time point 0, temperatures were slowly ramped up (0.5 °C per day) and the corals were cultured at 32 °C for >2 months. Graphs display the changes in colony surface covered by live tissue. The data points represent the average values. Error bars denote s.e.m. Significant values of P<0.05 or P<0.01 (two sample t-test) are indicated with one or two asterisks, respectively, below the corresponding experimental values. After an initial lag phase of ~3 weeks (dashed lines), the data could be fitted (full lines) as described in Supplementary Table S1. Representative micrographs of replicate colonies (lower panels) illustrate the time course of differential responses of strains 1 and 2.
The Indo-Pacific corals exhibited a contrasting behaviour to PAG specimens in these parallel treatments. They showed exponential growth under low-salinity conditions (Figures 4a and b), however, they ceased to grow or started to die after ~4 weeks at ambient temperature (27.5 °C) in the high-salinity treatment (Figures 4a and b). At elevated temperatures (32 °C), complete mortality of specimens kept at high salinity occurred ~2–3 weeks earlier compared to the treatment during which replicate colonies of the three species were exposed to high temperature at a salinity of 36.5 (Figure 4c). Under the latter conditions, the corals lost 90% of their live tissue in about 30–43 days. P. lobata–Symbiodinium C15 from Fiji has shown a comparable susceptibility to heat stress in a similar experiment (Hume et al., 2013).
Effect of salinity on the growth of Indo-Pacific corals. (a) Replicate colonies of M. foliosa and Montipora sp. were cultured at high (42) and reduced (36.5) salinity levels for >2 months. The wet weight of each fragment was determined at three time points during the incubation period. The data were normalised to the corresponding initial measurement values. Data points indicate changes in the average weight of five replicate colonies per species and condition, error bars denote s.e.m. Lines correspond to theoretical model fittings as detailed in Supplementary Table S1. The death of M. foliosa replicates before the last weight determination is indicated by a dashed line. Asterisks indicate significant difference between the treatments below the corresponding experimental values (two sample t-test, *P<0.05 and **P<0.01). (b and c) Effects of salinity and temperature stress on the survival of Indo-Pacific corals. Replicate colonies of M. foliosa, Montipora sp. and P. lichen corals were acclimated to two salinity levels (42 and 36.5) at a constant temperature of 27.5 °C. The temperature remained unaltered for the control corals over the duration of the >3-month experiment (b). At time point 0, temperatures were slowly ramped up (0.5 °C per day) for the treatment group and corals were incubated at 32 °C until the end of the experiment (c). The graphs display the changes in colony surface covered by live tissue. The data points represent the average of four and five colonies per genotype for the control and temperature treatments, respectively. Error bars denote s.e.m. Lines correspond to the theoretical models as detailed in Supplementary Table S1. M. foliosa samples exposed to Sal 42 under high-temperature stress died before the 16-day measurements, which is indicated by the dashed line section of the corresponding fitting.
Discussion
Distribution of the S. thermophilum group
Our present analysis reveals a striking predominance of Porites spp. symbioses with members of the S. thermophilum group in the southern PAG. These associations were found also in the Gulf of Oman, albeit at lower abundance. The steep decline in the frequency of C3-associated Porites spp. along the temperature and salinity gradient between the two waterbodies (Figure 1; Supplementary Figure S2) indicates that the high-salinity waters of the southern PAG represent the main distribution area of this unusual Porites–Symbiodinium symbiosis. In contrast, P. lobata and P. lutea populations from elsewhere in the world harbour almost exclusively Symbiodinium belonging to C15 cluster (rDNA ITS2 type). These findings suggest that the symbiosis with zooxanthellae of the S. thermophilum group is essential for coral survival in the extreme temperature and salinity environment of the southern PAG.
An uncommon association of Porites with Symbiodinium clade D was detected in some individuals collected in the transition zone between the PAG and the Gulf of Oman. Although some members of this symbiont clade are considered to increase the thermal tolerance of the holobiont among other coral species (Baker et al., 2004), our results show that it is not the basis for the thermal tolerance of Porites spp. under the prevailing environmental conditions of the southern PAG.
The gradual decline in the abundance of Porites spp./S. thermophilum group associations outside the PAG and the increasing dominance of the ‘cosmopolitan’ Porites–C15 associations indicate that the Porites/S. thermophilum partnership is—at least at the level of the holobiont—less successful outside of the extreme environment of the southern PAG. This is despite the fact that the temperature regime in the Gulf of Oman with large fluctuations and regular summer peaks of 32 °C is more challenging compared to most reef regions elsewhere (Riegl et al., 2011). Therefore, survival in the extreme environment of the PAG depends not only on temperature stress tolerance but also on the adaptation of multiple traits to local conditions that can constrain the physiological performance of the symbiotic association in other habitats. The change between the extraordinarily high salinity of the PAG and the normal oceanic salinity of the Gulf of Oman is well correlated with the decreasing prevalence of the association of Porites spp. with members of the S. thermophilum group, suggesting that salinity levels might have an important influence on the local adaptation of coral–alga symbioses. Indeed, we observed impaired growth and reduced heat tolerance of P. lobata/S. thermophilum associations at experimentally reduced salinity levels. Our findings suggest that the P. lobata/S. thermophilum symbiosis from the PAG is strongly adapted not only to the elevated temperatures but also to the exceptionally high salinity that characterises their local environment.
However, it is critical to note that although impaired by the reduced salinity, P. lobata/S. thermophilum endured heat stress for a longer period of time compared to the three species of Indo-Pacific corals tested in the present study and to P. lobata/Symbiodinium C15 from Fiji (Hume et al., 2013).
At present, it is not possible to discern whether local adaptation to temperature and salinity is dominated by physiological responses of the coral host or those of the algal symbionts. Although the host is likely to play an important role (Baird et al., 2009a), the prominent presence of S. thermophilum in the southern PAG (Hume et al., 2013; Hume et al., 2015) suggests that the symbionts make a decisive contribution. In fact, the only hint to a more widespread distribution of the S. thermophilum group exists in the form of a single database entry that reports a C3-Gulf ITS2 variant sequence from Stylophora pistillata from the Red Sea, an environment where temperatures and salinity can also reach exceptionally high levels on a regular basis (Hume et al., 2015). The importance of local adaptations of Symbiodinium types for the thermal tolerance of the coral holobiont has been also demonstrated for Acropora millepora from the Great Barrier Reef in Australia (Howells et al., 2012).
In agreement with the lower abundances of associations of Porites spp. with symbionts of the S. thermophilum group in the Gulf of Oman, our experiments confirmed that some corals associated with S. thermophilum can survive at normal oceanic salinity levels. However, the trade-offs resulting from local adaptation to deviating environmental conditions can strongly impair their physiological performance and offer a likely explanation for the constrained distribution of these symbiotic associations.
Implications for coral reef future and coral reef management
Despite the geographic isolation expected for PAG organisms (Sheppard et al., 1992), our results demonstrate that the distribution of symbionts of the S. thermophilum group, which are integral to coral survival in the world’s hottest seas, is not restricted to this water body. Instead, it can also be found in the adjacent Gulf of Oman where regular peak summer temperatures of 32 °C select for temperature stress-tolerant genotypes (Coles and Riegl, 2013; Riegl et al., 2012). The distribution across the Strait of Hormuz could potentially be promoted by the minor surface outflow of the PAG (Johns et al., 1999) that extends along the coast of Oman (Figure 1a) and runs in the opposite direction as the dominant inflow current (Sheppard et al., 1992). The Gulf of Oman might thus represent a ‘melting pot’ where traits such as temperature and salinity tolerance could be recombined, potentially producing genotypes that might be preadapted to the warmer oceans of the future. Global connectivity models (Wood et al., 2014) suggest that coral larvae with vertically transmitted symbionts (Baird et al., 2009b) might carry this genetic material from the Gulf of Oman into the Indian Ocean. Therefore, a high priority should be assigned to the protection of the marine environment in the PAG (Sale et al., 2011) and the Gulf of Oman.
Corals from the PAG have been discussed as subjects for assisted migration projects to protect them from the threat of rising temperature in their original habitat and to help to replenish reefs devastated elsewhere by global warming with heat-tolerant genotypes (Coles and Riegl, 2013). In addition to the general concerns about the feasibility and risks of long-range translocation and introduction of organisms into non-native environments (Coles and Riegl, 2013), our results suggest that due to the strong local adaptation, the superior stress tolerance of coral/S. thermophilum associations from the PAG might be reduced or even lost when they are exposed to the lower-salinity levels commonly found in the majority of reefs elsewhere, accordingly reducing the value of this conservation approach. Moreover, the weaker performance at lower salinity might contribute to an outbreeding depression (Aitken and Whitlock, 2013) in recipient populations. Further research is desirable to establish how other PAG coral/symbiont species would respond to changes in salinity. Meanwhile, coral reef management should not rely on long-range-assisted migration as a generally applicable solution for reefs devastated by global warming, but rather concentrate on alternative actions to strengthen the resilience of reef ecosystems.
Accession codes
References
Aitken SN, Whitlock MC . (2013). Assisted gene flow to facilitate local adaptation to climate change. Annu Rev Ecol Evol Syst 44: 367–388.
Baird AH, Bhagooli R, Ralph PJ, Takahashi S . (2009a). Coral bleaching: the role of the host. Trends Ecol Evol 24: 16–20.
Baird AH, Guest JR, Willis BL . (2009b). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40: 551–571.
Baker AC, Starger CJ, McClanahan TR, Glynn PW . (2004). Coral reefs: corals' adaptive response to climate change. Nature 430: 741.
Baker AC, Glynn PW, Riegl B . (2008). Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80: 435–471.
Burt J, Bartholomew A, Usseglio P . (2008). Recovery of corals a decade after a bleaching event in Dubai, United Arab Emirates. Mar Biol 154: 27–36.
Burt JA, Al-Khalifa K, Khalaf E, Alshuwaikh B, Abdulwahab A . (2013). The continuing decline of coral reefs in Bahrain. Mar Pollut Bull 72: 357–363.
Coles SL . (1992). Experimental comparison of salinity tolerances of reef corals from the Arabian Gulf and Hawaii: evidence for hyperhaline adaptation. Proc 7th Int Coral Reef Symp 1: 227–234.
Coles SL . (2003). Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: A comparison to the Indo-Pacific region. Atoll Res Bull 507: J1–J19.
Coles SL, Riegl BM . (2013). Thermal tolerances of reef corals in the Gulf: a review of the potential for increasing coral survival and adaptation to climate change through assisted translocation. Mar Pollut Bull 72: 323–332.
D'Angelo C, Wiedenmann J . (2012). An experimental mesocosm for long-term studies of reef corals. J Mar Biol Assoc UK 92: 769–775.
D’Angelo C, Wiedenmann J . (2014). Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr Opin Environ Sustain 7: 82–93.
Feary DA, Burt JA, Bauman AG, Al Hazeem S, Abdel-Moati MA, Al-Khalifa KA et al. (2013). Critical research needs for identifying future changes in Gulf coral reef ecosystems. Mar Pollut Bull 72: 406–416.
Ghavam Mostafavi P, Fatemi S, Shahhosseiny M, Hoegh-Guldberg O, Loh W . (2007). Predominance of clade D Symbiodinium in shallow-water reef-building corals off Kish and Larak Islands (Persian Gulf, Iran). Mar Biol 153: 25–34.
Goreau TF . (1964). Mass expulsion of zooxanthellae from Jamaican reef communities after Hurricane Flora. Science 145: 383–386.
Goreau TJ, Hayes RL . (1994). Coral bleaching and ocean ‘hot spots’. Ambio 23: 176–180.
Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, van Oppen MJH . (2012). Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat Clim Change 2: 116–120.
Hume B, D’Angelo C, Burt J, Baker A, Riegl B, Wiedenmann J . (2013). Corals from the Persian/Arabian Gulf as models for thermotolerant reef-builders: prevalence of clade C3 Symbiodinium, host fluorescence and ex situ temperature tolerance. Mar Pollut Bull 72: 313–322.
Hume BCC, D'Angelo C, Smith EG, Stevens JR, Burt J, Wiedenmann J . (2015). Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Sci Rep 5: 8562.
Johns WE, Jacobs GA, Kindle JC, Murray SP, Carron M . (1999). Arabian Marginal Seas and Gulfs, University of Miami RSMAS. Sponsored by the Office of Naval Research. Technical Report 2000–01: 60.
Lajeunesse TC . (2005). ‘Species’ radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22: 570–581.
LaJeunesse TC, Thornhill DJ . (2011). Improved resolution of reef-coral endosymbiont Symbiodinium species diversity, ecology, and evolution through psbA non-coding region genotyping. PLoS One 6: e29013.
LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA . (2014). Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) clade D are different species. Phycologia 53: 305–319.
Logan CA, Dunne JP, Eakin CM, Donner SD . (2014). Incorporating adaptive responses into future projections of coral bleaching. Global Change Biol 20: 125–139.
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R . (2001). Coral bleaching: the winners and the losers. Ecol Lett 4: 122–131.
Riegl B, Purkis S, Al-Cibahy A, Al-Harthi S, Grandcourt E, Al-Sulaiti K et al. (2012) Coral bleaching and mortality thresholds in the SE Gulf: highest in the world. In: Riegl BM, Purkis SJ (eds). Coral Reefs of the Gulf. Springer: : Netherlands, pp 95–105.
Riegl BM, Purkis SJ, Al-Cibahy AS, Abdel-Moati MA, Hoegh-Guldberg O . (2011). Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS One 6: e24802.
Sale P, Feary D, Burt J, Bauman A, Cavalcante G, Drouillard K et al. (2011). The growing need for sustainable ecological management of marine communities of the Persian Gulf. Ambio 40: 4–17.
Sheppard C, Loughland R . (2002). Coral mortality and recovery in response to increasing temperature in the southern Arabian Gulf. Aquat Ecosyst Health Manage 5: 395–402.
Sheppard CRC, Price ARG, Roberts CJ . (1992) Marine ecology of the Arabian area. Patterns and processes in extreme tropical environments. Academic Press: : London.
Thornhill DJ, Lewis AM, Wham DC, LaJeunesse TC . (2014). Host-specialist lineages dominate the adaptive radiation of reef coral endosymbionts. Evolution 68: 352–367.
van Hooidonk R, Maynard JA, Planes S . (2013). Temporary refugia for coral reefs in a warming world. Nat Clim Change 3: 508–511.
Wiedenmann J, D'Angelo C, Smith EG, Hunt AN, Legiret F-E, Postle AD et al. (2013). Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Change 3: 160–164.
Wood S, Paris CB, Ridgwell A, Hendy EJ . (2014). Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Global Ecol Biogeogr 23: 1–11.
Yao F, Johns WE . (2010). A HYCOM modeling study of the Persian Gulf: 1. Model configurations and surface circulation. J Geophys Res 115: C11017.
Acknowledgements
The study was funded by NERC (NE/K00641X/1 to JW), the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 311179 to JW and by a GSNOCS-CASE studentship to BCCH/JW. We thank the NYU Abu Dhabi Institute for supporting the 2012/2013 field workshops during which samples for this study were collected. Our appreciation is extended to A Al-Hemeri of the UAE Federal Environment Agency and A Al-Cibahy of the Environment Agency of Abu Dhabi for provision of CITES export permits (no. 09FEA555). We also thank Tropical Marine Centre (London) and Tropic Marin (Wartenberg) for sponsoring the NOCS Coral Reef Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
D'Angelo, C., Hume, B., Burt, J. et al. Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J 9, 2551–2560 (2015). https://doi.org/10.1038/ismej.2015.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2015.80
This article is cited by
-
Lineage-specific symbionts mediate differential coral responses to thermal stress
Microbiome (2023)
-
Mutualistic dinoflagellates with big disparities in ribosomal DNA variation may confound estimates of symbiont diversity and ecology in the jellyfish Cotylorhiza tuberculata
Symbiosis (2022)
-
Endosymbiotic Symbiodinium clades occurrence and influence on coral growth and resilience during stress
Symbiosis (2022)
-
Extending the natural adaptive capacity of coral holobionts
Nature Reviews Earth & Environment (2021)
-
The enhanced expression of heat stress-related genes in scleractinian coral ‘Porites harrisoni’ during warm episodes as an intrinsic mechanism for adaptation in ‘the Persian Gulf’
Coral Reefs (2021)