Abstract
Soil emissions are largely responsible for the increase of the potent greenhouse gas nitrous oxide (N2O) in the atmosphere and are generally attributed to the activity of nitrifying and denitrifying bacteria. However, the contribution of the recently discovered ammonia-oxidizing archaea (AOA) to N2O production from soil is unclear as is the mechanism by which they produce it. Here we investigate the potential of Nitrososphaera viennensis, the first pure culture of AOA from soil, to produce N2O and compare its activity with that of a marine AOA and an ammonia-oxidizing bacterium (AOB) from soil. N. viennensis produced N2O at a maximum yield of 0.09% N2O per molecule of nitrite under oxic growth conditions. N2O production rates of 4.6±0.6 amol N2O cell−1 h−1 and nitrification rates of 2.6±0.5 fmol NO2− cell−1 h−1 were in the same range as those of the AOB Nitrosospira multiformis and the marine AOA Nitrosopumilus maritimus grown under comparable conditions. In contrast to AOB, however, N2O production of the two archaeal strains did not increase when the oxygen concentration was reduced, suggesting that they are not capable of denitrification. In 15N-labeling experiments we provide evidence that both ammonium and nitrite contribute equally via hybrid N2O formation to the N2O produced by N. viennensis under all conditions tested. Our results suggest that archaea may contribute to N2O production in terrestrial ecosystems, however, they are not capable of nitrifier-denitrification and thus do not produce increasing amounts of the greenhouse gas when oxygen becomes limiting.
Similar content being viewed by others
Introduction
Nitrous oxide (N2O) is a greenhouse gas with 298 times the global warming potential of carbon dioxide over a 100-year period (IPCC, 2007). It contributes to the destruction of the stratospheric ozone layer (Conrad, 1996) and is even predicted to remain the dominant ozone-depleting substance of the twenty first century (Ravishankara et al., 2009). The increasing food demand of the human population has led to an excessive use of fertilizers in agriculture, which consequently increased N2O emissions considerably in the last century (Skiba and Smith, 2000; Galloway et al. 2008; Smith et al., 2012). As summed up by Smith et al. (2012) already in the year 2000 total N2O emissions accounted for 15.8 Tg N2O-N year−1, in which 5.6–6.5 Tg N2O-N year−1 could be assigned to an anthropogenic source and 4.3–5.8 Tg N2O-N year−1 to a land or coastal biological source.
The main processes responsible for gaseous nitrogen emissions from soil are microbial transformations of ammonium, nitrite, nitrate and to a lesser extent chemodenitrification (Colliver and Stephenson, 2000; Baggs, 2008, 2011; Campbell et al., 2011). Both ammonia-oxidizing and denitrifying microorganisms produce N2O by dissimilatory nitrate (or nitrite) reduction mostly under oxygen-limiting or anoxic conditions, whereas ammonia-oxidizing bacteria (AOB) can additionally produce N2O via hydroxylamine oxidation under oxic conditions, albeit to a lower extent (Hooper and Terry, 1979; Arp and Stein, 2003; Stein, 2011). It has been estimated that ammonia oxidizers can contribute considerably to direct terrestrial N2O emissions, depending on soil type and environmental conditions (Mummey et al., 1994; Webster and Hopkins, 1996; Gödde and Conrad, 1999; Pihlatie et al., 2004). In addition, they have an indirect influence on denitrification and thus N2O production through the production of the oxidized N-compound nitrite, the substrate for nitrite-oxidizing bacteria to produce nitrate, which in turn is used as a substrate by denitrifying microorganisms (Zhu et al., 2013).
The various N-transforming processes in soils that lead to N2O production are complex and the contributing microbial partners and environmental factors that influence its production are little understood (Baggs, 2011; Schreiber et al., 2012). For estimations or models of future greenhouse gas production and for the development of mitigation strategies it is therefore of great importance to identify all biological sources of N2O production and to characterize the environmental factors that influence their activity.
Recently, a novel group of ammonia oxidizers of the domain Archaea has been discovered to be widespread in marine and terrestrial environments, often outnumbering their bacterial counterparts by orders of magnitude (Leininger et al., 2006; Wuchter et al., 2006). The energy metabolism and general physiology of these ammonia-oxidizing archaea (AOA) is still not fully understood. Although it has been shown recently that hydroxylamine is an intermediate of ammonia oxidation in AOA (Vajrala et al., 2013) it has been argued that they might not have the capacity to produce N2O through a side reaction of ammonia oxidation, because they lack genes for a homolog of hydroxylamine oxidoreductase known to be responsible for N2O formation in AOB (Hooper and Terry, 1979) and in methanotrophic bacteria (Campbell et al., 2011). Furthermore, although AOA contain homologous genes of a nitrite reductase (NIR; Bartossek et al., 2010), they lack genes encoding a potential NO-reductase (NOR), which is involved in nitrifier-denitrification and thus N2O production in bacteria (Walker et al., 2010; Campbell et al., 2011; Stein, 2011; Tourna et al., 2011). Nevertheless, stable isotope-labeling experiments of marine enrichment cultures and measurements of a marine isolate have recently shown that AOA are indeed capable of N2O production (Santoro et al., 2011; Loescher et al., 2012). However, it has remained unclear under which conditions AOA produce N2O and if they are able to perform nitrifier-denitrification, the process that contributes most to direct N2O production of AOB in soils (Shaw et al., 2006). The characterization of the first AOA from soil obtained in a pure laboratory culture (Tourna et al., 2011) now allows studying the extent of N2O production in this group of organisms and to test the activity under varying environmental conditions, in particular under different oxygen concentrations. Here we present data from extensive laboratory incubations and a range of 15N-labeling experiments, designed to shed light on the mechanisms of formation and the environmental conditions under which N2O is produced by AOA.
Materials and methods
Strains and cultures
The AOA Nitrososphaera viennensis EN76 was maintained at 37 °C in fresh water medium according to Tourna et al. (2011). The AOA Nitrosopumilus maritimus SCM1 was incubated at 28 °C in SCM medium according to Könneke et al. (2005). N. viennensis and N. maritimus cultures were supplied with 1 mM ammonium and in addition with 0.1 mM pyruvate and 0.1 mM oxaloacetate, respectively. The media of N. viennensis and N. maritimus cultures were buffered with HEPES to a pH of 7.5. The AOB Nitrosospira multiformis ATCC 25196T (supplied by Jim Prosser, Aberdeen) was cultivated at 28 °C in Skinner and Walker (S+W) medium (Skinner and Walker, 1961) containing 1 mM ammonium and phenol red (0.5 mg) as pH indicator at a pH of 7.5–8. The pH was regularly adjusted by adding Na2CO3. Cultures were inoculated with 10% volumes of culture.
Growth was followed via photometric determination of ammonium consumption and nitrite production using a salicylic acid assay (Kandeler and Gerber, 1988) or a Grieß reagent system (Promega, Madison, WI, USA) for the latter. Screenings for contaminations were done regularly using light microscopy and PCR. Late exponential cultures were used to inoculate cultures for the determination of N2O production (10% inoculum), which have been set up in serum bottles (122 ml total; 20–30 ml medium; sealed with butyl rubber stoppers).
DNA extraction
Nucleic acids were extracted based on a modified protocol of Griffiths et al. (2000) using 2-ml Lysing Matrix E tubes (MP biomedicals, Eschwege, Germany) containing a mixture of silica, ceramic and glass beads in combination with the BIO101/Savant FastPrepFP120A Instrument (Qbiogene, Illkirch, France) for bead beating. Briefly, 1 ml of culture was harvested and the cell pellet was dissolved in 0.5 ml SDS extraction buffer (0.7 M NaCl, 0.1 M Na2SO3, 0.1 M Tris/HCl (pH 7.5), 0.05 M EDTA (pH8), 1% SDS). The further extraction was performed as described in the study by Nicol et al. (2005) with a DNA precipitation over night at 4 °C.
Quantitative PCR
Archaeal 16S rRNA genes were quantified using the primers Cren771F and Cren957R (Ochsenreiter et al., 2003). Amplification was performed in 20 μl reactions containing 10 μl QuantiFast SYBR Green PCR Mix (Qiagen, Hilden, Germany), 0.5 μM of each primer and 2 μl DNA template. For the standard curve a serial dilution of the linearized 16 S rRNA gene of N. viennensis was used with an efficiency of 101% and a slope of −3.3. The qPCR was performed in a realplex cycler (Mastercycler ep realplex, Eppendorf, Vienna, Austria) with the following PCR conditions: 95 °C for 15 min, 40 cycles of 30 s at 95 °C, 30 s at 54 °C and 30 s at 72 °C followed by a melting curve analysis at the end of the run to indicate the amplification of specific products. qPCR data were generated from independent DNA extractions of quadruplicate cultures with duplicated PCR amplifications.
N2O quantification
Cultures for the quantification of N2O were set up in replicates (3–5 cultures each) in serum bottles containing 20 ml fresh water medium. In addition, one blank with medium only and another one with dead cells (autoclaved culture) as inoculum were prepared. Production of N2O was tested under one fully aerated condition with 21% oxygen in the headspace and three oxygen limited conditions. To achieve this, reduced pressure was applied for 30 s followed by flushing with sterile filtered N2 (0% oxygen in headspace). To achieve a concentration of 10% and 3% oxygen in the gaseous phase a defined amount of N2 was replaced by sterile filtered air. Initial oxygen concentrations in the aqueous phase of the N. viennensis cultures (37 °C) were measured with an oxygen microsensor (Presens, Regensburg, Germany). Initial O2 concentrations in N. maritimus and N. multiformis cultures (28 °C) were calculated according to Henry’s law. Oxygen concentrations measured in the aqueous phase revealed that the aimed gaseous O2 concentrations were approximately achieved: 217±1 μM in the aqueous phase (corresponds to 21% O2 in gas phase), 114±4 μM (corresponds to 10% O2 in gas phase), 48±7 μM (corresponds to 3% O2 in gas phase) and 28±8 μM (corresponds to 0% O2 in gas phase). Owing to residual O2 dissolved in the medium measured values were slightly higher than expected.
Acetylene, an inhibitor of the ammonia monooxygenase, was added during exponential growth in a final concentration of 0.01%, which is sufficient to inhibit AOB as well as AOA (Hynes and Knowles, 1978; Offre et al., 2009).
Gas samples were taken at several time points during growth and 12 ml were transferred to 10-ml evacuated and sealed glass containers which were stored at 4 °C until analysis by GC (AGILENT 6890 N, Vienna, Austria; injector: 120 °C, detector: 350 °C, oven: 35 °C, carrier gas: N2) connected to an automatic sample-injection system (DANI HSS 86.50, Headspace-Sampler, Sprockhövel, Germany). N2O concentration was detected with a 63Ni-electron-capture detector. Standard gases (Inc. Linde Gas, Vienna, Austria) contained 0.5, 1 and 2.5 μl l−1 N2O. Further details are described elsewhere (Schaufler et al., 2010).
The removed gas in the cultures was replaced immediately by the respective gas phase (as described above; air, 10% and 3% O2 in N2 or only N2) in order to prevent reduced pressure. Furthermore, samples (220 μl) to determine nitrite and ammonium concentration were taken and analyzed photometrically as described above.
15N-labeling experiments
For N. viennensis, experiments with added 15NH4+ (1 mM 15NH4Cl; 5.05 at%) or 15NO2− (0.2 mM Na15NO2−; 9.69 at%) were carried out under oxic and oxygen-limited conditions (3% O2). In order to obtain comparable conditions between both 15N-labeling experiments we also added 0.2 mM of unlabeled NaNO2− to the cultures with 15NH4+-label. Bottles containing 0.2 mM NaNO2− and 1 mM NH4Cl but no inoculum were set up as media blanks. The precise isotopic composition of the label was determined by elemental analyzer (EA 1110, CE Instruments, Wigan, UK) coupled to an IRMS system (Finnigan ConFlo III interface and Finnigan DeltaPLUS isotope ratio mass spectrometer, Thermo Fisher, Vienna, Austria). Additionally, we performed an N2O isotope pool dilution assay for N. viennensis under oxygen-limited conditions (3% O2) by applying exogenously 15N-labeled N2O (∼300 nM, ∼49at%). 15N-labeled N2O was produced by the reduction of 15NO2− (98at%) to 15N2O by using azide (see below). Gross rates of N2O production and consumption were calculated based on isotope pool dilution theory (Kirkham and Bartholomew, 1954).
For all 15N-labeling experiments, we used serum bottles inoculated with 10% volumes of culture to a final volume of 30 ml. For each sampling during the growing phase (four times) quadruplicate bottles were prepared and triplicate un-inoculated media served as controls. We followed changes in concentration of NH4+, NO2− and N2O as well as isotopic composition of NO2− and N2O over time. Headspace samples were transferred to helium-flushed and pre-evacuated vials (12-ml exetainers) for N2O determination. Liquid samples for NH4+ and NO2− analysis were immediately frozen to −20 °C until used.
Concentrations of NH4+ and NO2− were measured as described above. Isotopic composition of NO2− was determined by a method based on the reduction of NO2− to N2O by using azide under acidified conditions following the protocol of Lachouani et al. (2010). Briefly, 1 ml sample or standard was transferred to 12-ml exetainer and 1 ml 1 M HCl was added. After purging the vials with helium to eliminate air-N2O in the sample headspace, 150 μl 1 M sodium azide buffer (in 10% acetic acid solution) were injected and the vials were placed on a shaker at 37 °C for 18 h. The reaction was stopped by injecting 250 μl of 10 M NaOH. For mass calibration, NO2− standards ranging from natural abundance to 8at% were analyzed. N2O concentration and isotopic ratio of the azide conversion as well as the headspace samples were determined using a purge-and-trap GC/IRMS system (PreCon, GasBench II headspace analyzer, Delta Advantage V; Thermo Fischer, Vienna, Austria). Isotopic ratios of N2O of the headspace samples were corrected for blanks.
Calculations
The N2O concentration was calculated per l culture at 25 °C and was corrected for the ambient concentration of N2O in air (or the respective gas atmosphere of the cultures). The N2O yield is the average ratio of μmol N2O per μmol NO2− produced and was generated from values of 2–4 time points during exponential growth. For the calculation of the nitrification rate (fmol cell−1 h−1) and N2O production rate (amol cell−1 h−1) the average cell density between two time points during exponential growth as approximated by qPCR was used.
The 15N-labeling experiments are closed systems where we can assume that there are only two possible sources for the formation of N2O: NH4+ (or an intermediate stemming from NH4+) and NO2−. We used a two-pool mixing model to determine the percentage contribution of each source pool to the product pool. Because of temporal changes in concentration and therefore in isotopic composition of NO2− through the input by ammonia oxidation calculations were performed for time intervals along the growing phase. The isotopic ratio of N2O produced in a certain time interval (Δ at %) was calculated as follows:

where Ct1, Ct2, at%t1 and at%t2 are N2O concentrations and atom% of N2O at t1 and t2 representing sampling time. ΔC is the increase in N2O concentration from t1 to t2. In this time interval the contribution of NO2− (source1) to N2O production was estimated by a two-pool mixing model:

where at%product is the isotopic ratio of N2O (according to Equation (1)). As the atom% of the NO2− pool changed in the course of time due to the input from ammonia oxidation we used the mean isotopic composition of NO2− between t1 and t2 as the atom% of NO2− (at%source1). At enrichment levels, as applied here, the discrimination between isotopes is negligible, and we therefore assumed that the isotopic composition of the NH4+ pool is constant over time. Thus, we used natural abundance (0.3663 atom%) and 5.05 atom% (which was determined as described above) for the unlabeled and labeled substrate addition experiments, respectively, as the source for N2O which derives from the NH4+ pool or an intermediate stemming from NH4+ (at%source2).
Following up on the results of the two-pool mixing model, we generated a probability model in order to distinguish whether the produced N2O was derived from hybrid formation or a combination of nitrifier-denitrification and ammonia oxidation. Owing to different labeled N sources (NH4+ and/or NO2−) different N2O-forming processes will yield a distinctive fraction of N2O, which is double-labeled (15N15NO). Therefore, the model predicts the concentration of double-labeled N2O (15N15NO) as a function of N2O concentration (that is, sum of N2O with mass 44, 45 and 46). It is based on the theoretical probability of the occurrence of N2O with mass 46 (46N2O; including natural abundance of oxygen isotopes). The probability of the occurrence of 46N2O is the sum of the probabilities of four isotopologs:

To determine the probability of each isotopolog, we multiplied the respective relative natural abundance of the O isotope (16O, 17O or 18O), the relative 15N abundance [P(15N)] and/or the relative abundance of 14N of the N source [P(14N)=1−P(15N)]. In the case that N2O is solely produced during ammonia oxidation, P(46N2O) is based on the isotopic composition of the NH4+ pool. Assuming that only nitrifier-denitrification occurs, P(46N2O) is calculated from the isotopic composition of the NO2− pool. If those two processes occur simultaneously, P(46N2O) is the sum of their relative contributions. In case of hybrid N2O formation (that is, one N atom stems from NO2− and one from NH4+ or an intermediate of ammonia oxidation), the model considers that one N atom of each isotopolog derives from NH4+ and the other one from NO2−, which are combined to form hybrid N2O. For the 15NO2−-labeling experiments, we computed P(46N2O) for the different scenarios based on the isotopic composition of NH4+, which was constant at natural abundance, and NO2−, which varied between ∼2–4.4 at% due to input by ammonia oxidation. We accounted for this variability in the NO2− isotopic composition by considering the 15N relative abundance of the NO2− pool as a function of N2O concentration. For each scenario using the respective P(46N2O) function, we calculated the cumulative 46N2O concentration (that is, 15N15NO) as a function of N2O produced according to Equation 4.

where x is the N2O concentration, xt0 is the N2O concentration at the first sampling time and 46N2Oxt0 is the concentration of 46N2O at the first sampling time.
Statistical analyses
In case of using independent variables for calculation (that is, independent samples of two samplings during the growth phase) the standard error was estimated by propagation of error. Analysis of variance, Holm–Sidak post hoc tests and t-tests (α=0.05) were performed using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA, USA).
Results
Quantification of N2O production
N2O production was measured in N. viennensis cultures amended with different initial headspace oxygen concentrations (21%, 10%, 3% and 0%) and compared with N2O production from the AOA N. maritimus and the AOB N. multiformis, grown under the same initial ammonia and oxygen concentrations. N2O accumulation, nitrite production and ammonia consumption are shown in Figure 1.
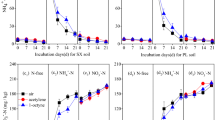
Near stoichiometric conversion of ammonium (squares, dotted lines) to nitrite (triangles, solid lines; upper plots) and concurrent N2O production (circles; lower plots) during the growth of the AOA N. viennensis and N. maritimus as well as the AOB N. multiformis. The strains have been cultivated under four different oxygen concentrations (21%: black; 10%: dark gray; 3%: light gray; 0%: white). Mean values of triplicate or quadruplicate experiments, respectively, are shown with standard deviations plotted.
N2O production paralleled nitrite production in all strains over the incubation period. N2O production of N. viennensis and N. maritimus was dependent on ammonia oxidation and was not significantly affected by the varying oxygen concentrations (Figure 1, Supplementary Table S1) with stable N2O yields at all tested oxygen concentrations. There was no increase in N2O production with decreasing oxygen. In contrast, both strains reached slightly higher maximal N2O concentrations and yields at higher oxygen concentrations (Table 1). Independent of the oxygen concentration N. viennensis produced almost twice as much N2O as N. maritimus. For example, N. viennensis had a maximal yield of 0.09 (±0.00) % N2O/NO2− and a maximal N2O concentration of 0.80 (±0.08) μM N2O at 21% O2 in the headspace, whereas N. maritimus produced at maximum 0.44 (±0.04) μM N2O with an N2O yield of 0.05 (±0.02) % N2O/NO2− at the same oxygen level.
Different from that of N. viennensis and N. maritimus, N2O production and yields of the AOB N. multiformis increased 3–4-fold under decreasing oxygen concentrations, which is in line with earlier studies (Goreau et al., 1980; Anderson and Levine, 1986). N. multiformis showed a maximal N2O production of 3.32 (±0.30) μM N2O and the highest N2O yield of 0.27 (±0.05) % N2O/NO2− under 0% O2 in the headspace. In comparison with both AOA the maximal N2O production of N. multiformis (AOB) was significantly higher at all tested oxygen concentrations (see Supplementary Table S1 for statistical tests).
Nitrification and N2O production rates were determined for N. viennensis by relating production to cell numbers estimated by quantitative PCR of the 16S rRNA gene, which occurs only once in the genome (Tourna et al., 2011). The N2O production rates were 4.6 (±0.6) amol cell−1 h−1 under ambient oxygen and 4.2 (±0.1) amol cell−1 h−1 under reduced oxygen (3% O2 in headspace), with nitrification rates of 2.6 (±0.5) and 2.8 (±0.5) fmol nitrite cell−1 h−1, respectively (Table 2).
When 0.01% of the ammonia oxidation inhibitor acetylene was supplied to an exponentially growing culture of N. viennensis, both nitrite production and N2O production ceased immediately, indicating that N2O production was linked to the process of ammonia oxidation as has been shown for AOB (Supplementary Figure S1). Furthermore, controls with inactivated cells or media blanks without cell inoculum but supplemented with nitrite did not show any increase in N2O concentration over the incubation period (not shown).
Contribution of ammonia-N and nitrite-N to N2O
To elucidate the potential mechanism of N2O production in N. viennensis we conducted 15N-labeling experiments using either 15N-labeled ammonium plus unlabeled nitrite or vice versa. When exogenous 15NH4+ was supplied, a continuous increase in the 15N/14N ratio of the NO2− pool over time was observed, reflecting the enrichment of labeled NO2− from ammonia oxidation (Figures 2a and b and Supplementary Figures S2A and B). The 15N/14N ratio of the concurrently produced N2O was higher compared with NO2− throughout the experiment. The addition of 15N-labeled NO2− (together with unlabeled NH4+) resulted in a decrease of the 15N/14N ratio of the NO2− pool over time due to the input of unlabeled NO2− from ammonia oxidation (Figures 2c and d and Supplementary Figures S2C and D). In this case the concurrently produced N2O had a lower 15N/14N ratio compared with NO2− at both oxygen concentrations. Thus, in both labeling experiments the differences between the isotopic composition of NO2− and N2O indicated that both NO2− and NH4+ contributed to the production of N2O.
15N-labeling experiment of N. viennensis under ambient oxygen concentration (21%) with an addition of 15NH4+ (a, b; 1mM 15NH4+ and 0.2mM 14NO2−) and 15NO2− (c and d; 0.2mM 15NO2− and 1mM 14NH4+). The concentrations of NH4+, NO2− and N2O were followed during the growth phase (a, c). The NO2− concentrations presented here were corrected for the exogenously supplied NO2−. The isotopic composition of NO2− and N2O are atom percent excess (APE; b, d). When 15NO2− was exogenously supplied, no label was recovered as NH4+. At each sampling day samples were harvested from independent flasks. Each data point represents the mean value of four replicates (±1 s.e.).
The 15N-labeling experiments were closed system experiments with only two possible N-sources for the formation of N2O: NH4+ (or an intermediate of ammonia oxidation stemming from NH4+) and NO2−. Therefore, a two-pool mixing model was used to elucidate the contribution of NO2− to the formation of N2O. The contribution of NO2− to the formation of N2O under ambient oxygen concentrations was 40.2% and 40.8% in the 15NH4+ and 15NO2−-labeling experiments, respectively, and under reduced oxygen conditions 46.6% and 45.1%, respectively (Figure 3a). These results show a nearly equal contribution of NH4+ and NO2− to the N2O production at both oxygen levels tested.
Two-pool mixing model (a) showing the comparison of percentage contribution of NO2− to the N2O formation between 15N-labeling experiments (addition of 15NH4+ and 15NO2−) at each O2-treatment (21% and 3%) for N. viennensis. Data presented are means of all time intervals along the growth phase for each experiment (± 1s.e.). We found no significant difference in the mean of the percentage contribution of NO2− to the N2O formation between 15NH4+- and 15NO2−-labeling experiments at each O2 treatment (t-test, 21% oxygen, t4=−0.0868, P=0.935; 3% oxygen, t4=0.167, P=0.876). Within each labeling experiment, there is also no significant difference between the mean of the O2-treatments (t-test, 15NH4+-labeling, t4=−0.677, P=0.536; 15NO2−-labeling, t4=−0.810, P=0.463). Probability models (b, c) showing predicted double-labeled N2O (15N15NO; based on the theoretical probability of the occurrence of N2O with mass 46) produced by different possible pathways compared with measured data of the 15NO2−-labeling experiment under oxic (b) and reduced oxygen (C; 3% O2 in headspace) conditions. The grey shaded area represents a combination of ammonia oxidation and nitrifier-denitrification with a contribution of each process between 40 and 60%. The upper border of the grey shaded area represents a contribution of nitrifier-denitrification with 60% and ammonia oxidation with 40% and vice versa for the lower border.
We found no significant difference in the contribution of NO2− to N2O between ambient and reduced oxygen condition, which was corroborated by two independent 15N-labeling approaches with 15NH4+ and 15NO2−.
In order to distinguish whether the produced N2O by N. viennensis was derived from hybrid formation (that is, one N atom stems from NO2− and one from NH4+ or an intermediate of ammonia oxidation) or a combination of two simultaneous processes (that is, nitrifier-denitrification and ammonia oxidation) we calculated the concentration of double-labeled N2O (15N15NO) for different N2O-forming processes. Each process, or a combination of them, will yield a distinctive fraction of double-labeled N2O (46N2O). The probability model shows that a combination of nitrifier-denitrification and ammonia oxidation with a relative contribution between 40 and 60% as indicated by the two-pool mixing model was unlikely, under both oxygen conditions tested (Figures 3b and c). The model fitted to the measured data suggests under oxic conditions a relative contribution of ∼20% and 80% by nitrifier-denitrification and ammonia oxidation, respectively, and of ∼14% and 86% under reduced oxygen conditions, which stands in contrast to the results of the two-pool mixing model. The results of the probability model point to N2O production via hybrid formation or only via ammonia oxidation, whereas the latter case can be excluded because we detected 15N2O while labeling the nitrite pool. Taken together, the results of the two-pool mixing model and the probability model indicate hybrid N2O formation by N. viennensis.
To clarify whether the produced N2O was further metabolized, for example to N2, we used an isotope pool dilution assay in which we labeled the N2O pool with 15N2O enabling us to calculate gross and net production rates of the greenhouse gas under oxygen-limiting conditions in N. viennensis. The gross N2O production (14.4 nmol l−1 h−1±1.1) was not significantly different from net N2O production (12.1 nmol l−1 h−1±1.2), indicating that N2O was neither reduced further to N2 nor re-assimilated.
Discussion
The growth of N. viennensis in pure culture allowed us to determine, for the first time, gross nitrification and N2O production rates of an AOA from soil.
Despite its relatively small cell size, N2O production rates (4.2–4.6 amol N2O-N h−1 cell−1) of N. viennensis were comparable to many bacterial ammonia-oxidizing soil strains (N. multiformis ATCC 25196: 7.6 amol N2O-N h−1 cell−1, Nitrosospira sp. strain 40KI: 4.6 amol N2O-N h−1 cell−1, Nitrosospira sp. strain NpAV: 3.9 amol N2O-N h−1 cell−1 (Shaw et al., 2006)) but lower than those measured for strains of the genus Nitrosomonas (Goreau et al., 1980; Hynes and Knowles, 1984; Anderson and Levine, 1986; Remde and Conrad, 1990; Shaw et al., 2006). Similarly, molar yields of N2O (expressed as a percentage of moles of NO2− produced) were of the same order of magnitude as those reported for many AOB (Jiang and Bakken, 1999; Shaw et al., 2006).
N2O yields of N. viennensis (0.07–0.09% N2O/NO2−) were higher compared with group I.1a AOA enrichment cultures (0.0022–0.055% N2O/NO2− (Santoro et al., 2011), 0.065% N2O/NO2− (Jung et al., 2011)) and to the pure culture of N. maritimus (0.03–0.05% N2O/NO2−(this study), 0.002–0.026% N2O/NH4+ (Loescher et al., 2012)). The measured N2O yields for N. maritimus were thus in accordance with reported values and slightly higher than reported earlier (Loescher et al., 2012). However, Loescher et al. (2012) have obtained maximal N2O yields for N. maritimus under limited oxygen concentrations (initial concentration: 112 μM O2; in line with 10% O2 culture in our study) and reported decreasing N2O concentrations with increasing oxygen levels. At ambient oxygen concentrations Loescher et al. (2012) have shown 20 times lower N2O yields for N. maritimus compared with yields obtained in our study for the same organism. In this study, we could not observe a significant difference (P<0.001) in maximal N2O concentrations produced by N. maritimus at the four different oxygen concentrations tested. Different from the study by Loescher et al. (2012) we have added oxaloacetate to the culture medium, which led to an increased growth rate and also higher cell numbers for N. maritimus and this might have also caused higher N2O production.
The equally high production of N2O by N. viennensis and N. maritimus under different oxygen levels and especially the lack of an increase in N2O production under oxygen limitation indicate that AOA are not capable of nitrifier-denitrification (N2O production from nitrite alone). This is supported by the absence of genes for bona fide nitric oxide reductase (NOR) in the genomes of AOA (Walker et al., 2010; Tourna et al., 2011; Spang et al., 2012) and is also in agreement with earlier isotopic studies in which the site preferences of N2O indicated that it is mainly not produced via nitrifier-denitrification (Santoro et al., 2011; Loescher et al., 2012).
Hybrid N2O formation in N. viennensis
Stable isotope-labeling experiments with N. viennensis showed a nearly equal contribution of nitrogen from ammonia and nitrite to the N2O production at all oxygen levels tested. This was further supported, when we modeled the amount of dually labeled N2O molecules (15N-15N-O) to determine the different possible pathways that could be used to synthesize N2O and compared the calculated values to the actual measurements obtained from our experiments with 15N-labeled nitrite under ambient and reduced oxygen concentrations (Figures 3b and c). Thus, N. viennensis seems to produce N2O during aerobic ammonia oxidation, from nitrite and an intermediate of ammonia oxidation mostly via a hybrid formation mechanism. Such a mechanism of N2O formation is also known from denitrifying fungi and bacteria mainly under anoxic or reduced oxygen conditions, where it is described as co-metabolic denitrification (that is, co-denitrification) through a biotically mediated N-nitrosation reaction (Spott et al., 2011). In this process one N from nitrite or NO is combined in an enzymatic reaction with one N from a co-substrate (ammonium, hydroxylamine, amines, and so on). NIR and NOR have been suggested as possible enzyme candidates catalyzing this reaction (Spott et al., 2011). As all published thaumarchaeal genomes (except that of Cenarchaeum symbiosum) contain a nirK homolog (encoding NIR) (Bartossek et al., 2010) and as it has been shown in metatranscriptomic studies that this thaumarchaeal gene is highly expressed in planktonic samples (Frias-Lopez et al., 2008; Hollibaugh et al., 2011), sponge tissues (Radax et al., 2012) and in soil (Urich et al., 2008), it might be a good candidate for performing this reaction. However, one has to note that the term co-denitrification has so far been used for a process that increases with decreasing oxygen concentrations (Spott et al., 2011), which was not the case for N2O production in our AOA study.
There are two main N2O production mechanisms described for bacterial ammonia oxidizers. Under oxic conditions AOB oxidize hydroxylamine by hydroxylamine oxidoreductase to NO, which is further oxidized to N2O by a yet unknown enzyme (Hooper and Terry, 1979; Schreiber et al., 2012). However, cytochrome c554 and NorS have been discussed as potential candidates for this reaction in AOB (Stein, 2011), whereas CytS has been described to have a role in NO-detoxification in methane-oxidizing bacteria (Poret-Peterson et al., 2008; Campbell et al., 2011). Under reduced oxygen conditions N2O is produced via the process of nitrifier-denitrification, which is the reduction of nitrite to NO by NIR and a further reduction to N2O by NOR (Goreau et al., 1980; Arp and Stein, 2003). However, some nitrifier-denitrification of AOB has also been demonstrated under oxic conditions (Shaw et al., 2006). In addition, it has been discussed that aerobic N2O production in AOB might proceed via a different and unknown pathway including HNO as a further intermediate of ammonia oxidation, which might react abiotically to N2O (Schreiber et al., 2012). Further, a recent study has shown the emission of HONO and NO by the AOB Nitrosomonas europaea (Oswald et al., 2013).
The pathway of ammonia oxidation in AOA is still not fully understood. Although it has been shown recently that hydroxylamine is an intermediate of ammonia oxidation in N. maritimus (Vajrala et al., 2013) homologous genes of hydroxylamine oxidoreductase have not been identified in their genomes (Walker et al., 2010; Kim et al., 2011; Tourna et al., 2011; Spang et al., 2012). Thus, biotic production of hybrid N2O via the yet unidentified second enzyme of ammonia oxidation cannot be excluded. However, we can also not exclude an abiotic formation of hybrid N2O via an N-nitrosation reaction of nitrite and an intermediate of ammonia oxidation, for example, hydroxylamine, HNO or NO (Zollinger, 1988; Spott et al., 2011), which have been discussed to be possible intermediates of AOA (Schleper and Nicol, 2010; Walker et al., 2010; Vajrala et al., 2013). Noteworthy, recent studies by Yan et al. (2012) and our laboratory (Shen et al., 2013) have demonstrated inhibition of ammonia oxidation by carboxy-PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide), a scavenger of NO (Amano and Noda, 1995; Akaike and Maeda, 1996), indicating that NO does indeed have an important role in the energy metabolism of AOA as postulated earlier (Schleper and Nicol, 2010; Walker et al., 2010).
Conclusion
N. viennensis and N. maritimus, the only available pure cultures of AOA, produced N2O under oxic conditions at similar yields and rates as bacterial ammonia oxidizers grown under similar conditions (for example, same ammonia supply). However, both AOA are not capable of nitrifier-denitrification like AOB and thus do not produce increasing amounts of the greenhouse gas when oxygen becomes limiting. 15N-labeling studies performed with N. viennensis indicate N2O production that results in hybrid formation independent of the oxygen concentration.
Extrapolating from our data obtained with two representatives of the two major clades of AOA (soil and marine clade) and considering the vast numbers of AOA (Karner et al., 2001; Leininger et al., 2006; Wuchter et al., 2006; Adair and Schwartz, 2008; Shen et al., 2008) and their ammonia-oxidizing activity in both terrestrial and oceanic environments (Martens-Habbena et al., 2009; Offre et al., 2009; Di et al., 2010; Verhamme et al., 2011) one can assume that AOA contribute directly to continuous persistent N2O emissions, albeit at low rates, comparable to those of AOB under oxic conditions and low ammonia supply. As AOB might produce more N2O under higher ammonia concentrations than supplied in our experiments their relative contribution to N2O emissions in the environment is certainly higher than that of AOA on a per-cell basis. A bigger contribution to global N2O production through AOA might occur rather indirectly through the production of oxidized nitrogenous compounds (mostly NO2−) that are converted into substrates for denitrifying organisms.
References
Adair KL, Schwartz E . (2008). Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microb Ecol 56: 420–426.
Akaike T, Maeda H . (1996). Quantitation of nitric oxide using 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO). Methods Enzymol 268: 211–221.
Amano F, Noda T . (1995). Improved detection of nitric oxide radical (NO.) production in an activated macrophage culture with a radical scavenger, carboxy PTIO and Griess reagent. FEBS Lett 368: 425–428.
Anderson IC, Levine JS . (1986). Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers. Appl Environ Microbiol 51: 938–945.
Arp DJ, Stein LY . (2003). Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit Rev Biochem Mol Biol 38: 471–495.
Baggs EM . (2008). A review of stable isotope techniques for N2O source partitioning in soils: recent progress, remaining challenges and future considerations. Rapid Commun Mass Spectrom 22: 1664–1672.
Baggs EM . (2011). Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Curr Opin Environ Sustainability 3: 321–327.
Bartossek R, Nicol GW, Lanzen A, Klenk HP, Schleper C . (2010). Homologues of nitrite reductases in ammonia-oxidizing archaea: diversity and genomic context. Environ Microbiol 12: 1075–1088.
Campbell MA, Nyerges G, Kozlowski JA, Poret-Peterson AT, Stein LY, Klotz MG . (2011). Model of the molecular basis for hydroxylamine oxidation and nitrous oxide production in methanotrophic bacteria. FEMS Microbiol Lett 322: 82–89.
Colliver BB, Stephenson T . (2000). Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol Adv 18: 219–232.
Conrad R . (1996). Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60: 609–640.
Di HJ, Cameron KC, Shen JP, Winefield CS, O'Callaghan M, Bowatte S et al. (2010). Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72: 386–394.
Frias-Lopez J, Shi Y, Tyson GW, Coleman ML, Schuster SC, Chisholm SW et al. (2008). Microbial community gene expression in ocean surface waters. Proc Natl Acad Sci USA 105: 3805–3810.
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR et al. (2008). Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320: 889–892.
Gödde M, Conrad R . (1999). Immediate and adaptational temperature effects on nitric oxide production and nitrous oxide release from nitrification and denitrification in two soils. Biol Fert Soil 30: 33–40.
Goreau TJ, Kaplan WA, Wofsy SC, Mcelroy MB, Valois FW, Watson SW . (1980). Production of No2- and N2o by nitrifying bacteria at reduced concentrations of oxygen. Appl Environ Microbiol 40: 526–532.
Griffiths RI, Whiteley AS, O'Donnell AG, Bailey MJ . (2000). Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66: 5488–5491.
Hollibaugh JT, Gifford S, Sharma S, Bano N, Moran MA . (2011). Metatranscriptomic analysis of ammonia-oxidizing organisms in an estuarine bacterioplankton assemblage. ISME J 5: 866–878.
Hooper AB, Terry KR . (1979). Hydroxylamine oxidoreductase of nitrosomonas production of nitric-oxide from hydroxylamine. Biochim Biophys Acta 571: 12–20.
Hynes RK, Knowles R . (1978). Inhibition by acetylene of ammonia oxidation in Nitrosomonas-Europaea. FEMS Microbiol Lett 4: 319–321.
Hynes RK, Knowles R . (1984). Production of nitrous-oxide by Nitrosomonas-Europaea—effects of acetylene, pH, and oxygen. Can J Microbiol 30: 1397–1404.
IPCC (2007). Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Chapter 2.2. Cambridge University Press: Cambridge.
Jiang QQ, Bakken LR . (1999). Nitrous oxide production and methane oxidation by different ammonia-oxidizing bacteria. Appl Environ Microbiol 65: 2679–2684.
Jung MY, Park SJ, Min D, Kim JS, Rijpstra WI, Sinninghe Damste JS et al. (2011). Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microbiol 77: 8635–8647.
Kandeler E, Gerber H . (1988). Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soils 6: 68–72.
Karner MB, DeLong EF, Karl DM . (2001). Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409: 507–510.
Kim BK, Jung MY, Yu DS, Park SJ, Oh TK, Rhee SK et al. (2011). Genome sequence of an ammonia-oxidizing soil archaeon, ‘Candidatus Nitrosoarchaeum koreensis’ MY1. J Bacteriol 193: 5539–5540.
Kirkham DON, Bartholomew WV . (1954). Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci Soc Amer Proc 18: 33–34.
Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA . (2005). Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437: 543–546.
Lachouani P, Frank AH, Wanek W . (2010). A suite of sensitive chemical methods to determine the δ15N of ammonium, nitrate and total dissolved N in soil extracts. Rapid Commun Mass Spectrom 24: 3615–3623.
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW et al. (2006). Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442: 806–809.
Loescher CR, Kock A, Koenneke M, LaRoche J, Bange HW, Schmitz RA . (2012). Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences Discuss 9: 2095–2122.
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA . (2009). Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461: 976–979.
Mosier AC, Allen EE, Kim M, Ferriera S, Francis CA . (2012a). Genome sequence of ‘Candidatus Nitrosoarchaeum limnia’ BG20, a low-salinity ammonia-oxidizing archaeon from the San Francisco Bay estuary. J Bacteriol 194: 2119–2120.
Mosier AC, Allen EE, Kim M, Ferriera S, Francis CA . (2012b). Genome sequence of ‘Candidatus Nitrosopumilus salaria’ BD31, an ammonia-oxidizing archaeon from the San Francisco Bay estuary. J Bacteriol 194: 2121–2122.
Mummey DL, Smith JL, Bolton H Jr . (1994). Nitrous oxide flux from a shrub-steppe ecosystem: sources and regulation. Soil Biol Biochem 26: 279–286.
Nicol GW, Tscherko D, Embley TM, Prosser JI . (2005). Primary succession of soil Crenarchaeota across a receding glacier foreland. Environ Microbiol 7: 337–347.
Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C . (2003). Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5: 787–797.
Offre P, Prosser JI, Nicol GW . (2009). Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. Fems Microbiol Ecol 70: 99–108.
Oswald R, Behrendt T, Ermel M, Wu D, Su H, Cheng Y et al. (2013). HONO emissions from soil bacteria as a major source of atmospheric reactive nitrogen. Science 341: 1233–1235.
Pihlatie M, Syvasalo E, Simojoki A, Esala M, Regina K . (2004). Contribution of nitrification and denitrification to N2O production in peat, clay and loamy sand soils under different soil moisture conditions. Nutr Cycling Agroecosyst 70: 135–141.
Poret-Peterson AT, Graham JE, Gulledge J, Klotz MG . (2008). Transcription of nitrification genes by the methane-oxidizing bacterium, Methylococcus capsulatus strain Bath. ISME J 2: 1213–1220.
Radax R, Rattei T, Lanzen A, Bayer C, Rapp HT, Urich T et al. (2012). Metatranscriptomics of the marine sponge Geodia barretti: tackling phylogeny and function of its microbial community. Environ Microbiol 14: 1308–1324.
Ravishankara AR, Daniel JS, Portmann RW . (2009). Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326: 123–125.
Remde A, Conrad R . (1990). production of nitric-oxide in nitrosomonas-europaea by reduction of nitrite. Arch Microbiol 154: 187–191.
Santoro AE, Buchwald C, McIlvin MR, Casciotti KL . (2011). Isotopic signature of N(2)O produced by marine ammonia-oxidizing archaea. Science 333: 1282–1285.
Schaufler G, Kitzler B, Schindlbacher A, Skiba U, Sutton MA, Zechmeister-Boltenstern S . (2010). Greenhouse gas emissions from European soils under different land use: effects of soil moisture and temperature. Eur J Soil Sci 61: 683–696.
Schleper C, Nicol GW . (2010). Ammonia-oxidising archaea—physiology, ecology and evolution. Adv Microb Physiol 57: 1–41.
Schreiber F, Wunderlin P, Udert KM, Wells GF . (2012). Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions and novel technologies. Front Microbiol 3: 372.
Shaw LJ, Nicol GW, Smith Z, Fear J, Prosser JI, Baggs EM . (2006). Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ Microbiol 8: 214–222.
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ . (2008). Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10: 1601–1611.
Shen T, Stieglmeier M, Dai J, Urich T, Schleper C . (2013). Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344: 121–129.
Skiba U, Smith KA . (2000). The control of nitrous oxide emissions from agricultural and natural soils. Chemosphere Global Change Sci 2: 379–386.
Skinner FA, Walker N . (1961). Growth of Nitrosomonas europaea in batch and continuous culture. Arch Microbiol 38: 339–349.
Smith KA, Mosier AR, Crutzen PJ, Winiwarter W . (2012). The role of N2O derived from crop-based biofuels, and from agriculture in general, in Earth's climate. Philos Trans R Soc B Biol Sci 367: 1169–1174.
Spang A, Poehlein A, Offre P, Zumbragel S, Haider S, Rychlik N et al. (2012). The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: insights into metabolic versatility and environmental adaptations. Environ Microbiol 14: 3122–3145.
Spott O, Russow R, Stange CF . (2011). Formation of hybrid N2O and hybrid N-2 due to codenitrification: first review of a barely considered process of microbially mediated N-nitrosation. Soil Biol Biochem 43: 1995–2011.
Stein LY . (2011). Surveying N2O-producing pathways in bacteria. Methods Enzymol 486: 131–152.
Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T et al. (2011). Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108: 8420–8425.
Urich T, Lanzen A, Qi J, Huson DH, Schleper C, Schuster SC . (2008). Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS One 3: e2527.
Vajrala N, Martens-Habbena W, Sayavedra-Soto LA, Schauer A, Bottomley PJ, Stahl DA et al. (2013). Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci USA 110: 1006–1011.
Verhamme DT, Prosser JI, Nicol GW . (2011). Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5: 1067–1071.
Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ et al. (2010). Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107: 8818–8823.
Webster FA, Hopkins DW . (1996). Contributions from different microbial processes to N2O emission from soil under different moisture regimes. Biol Fert Soils 22: 331–335.
Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P et al. (2006). Archaeal nitrification in the ocean. Proc Natl Acad Sci USA 103: 12317–12322.
Yan J, Haaijer SC, Op den Camp HJ, van Niftrik L, Stahl DA, Konneke M et al. (2012). Mimicking the oxygen minimum zones: stimulating interaction of aerobic archaeal and anaerobic bacterial ammonia oxidizers in a laboratory-scale model system. Environ Microbiol 14: 3146–3158.
Zhu X, Burger M, Doane TA, Horwath WR . (2013). Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci USA 110: 6328–6333.
Zollinger H . (1988). Diazotizations in highly concentrated mineral acids—the nitrosation mechanism of anilinium and hydroxylammonium ions through proton loss from the ammonio group. Helv Chim Acta 71: 1661–1664.
Acknowledgements
We thank Daniela Teichmann, Romana Bittner, Michaela Djordjevic and Margarete Watzka for technical assistance, Daniel Schenz for help with data analysis, Rebecca Hood-Nowotny and Ricardo E Alves for critically reading the manuscript and Jim Prosser (Aberdeen) for supply of strain N. multiformis. This work was supported by the University of Vienna, Faculty of Life Sciences, and by FWF (Austrian Science Fund) grant P23000 to CS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Stieglmeier, M., Mooshammer, M., Kitzler, B. et al. Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J 8, 1135–1146 (2014). https://doi.org/10.1038/ismej.2013.220
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2013.220
Keywords
This article is cited by
-
Direct biological fixation provides a freshwater sink for N2O
Nature Communications (2023)
-
Nitrification and beyond: metabolic versatility of ammonia oxidising archaea
The ISME Journal (2023)
-
Effects of acidification on nitrification and associated nitrous oxide emission in estuarine and coastal waters
Nature Communications (2023)
-
Saltwater incursion regulates N2O emission pathways and potential nitrification and denitrification in intertidal wetland
Biology and Fertility of Soils (2023)
-
Greenhouse gas fluxes from different types of permafrost regions in the Daxing'an Mountains, Northeast China
Environmental Science and Pollution Research (2023)