Abstract
Microorganisms are globally dispersed and are able to proliferate in any habitat that supports their lifestyles, which, however, has not yet been explored in any specific microbial taxon. The social myxobacteria are considered typical soil bacteria because they have been identified in various terrestrial samples, a few in coastal areas, but none in other oceanic environments. To explore the prevalence of marine myxobacteria and to investigate their phylogenetic relationships with their terrestrial counterparts, we established myxobacteria-enriched libraries of 16S rRNA gene sequences from four deep-sea sediments collected at depths from 853 to 4675 m and a hydrothermal vent at a depth of 204 m. In all, 68 different myxobacteria-related sequences were identified from randomly sequenced clones of the libraries of different samples. These myxobacterial sequences were diverse but phylogenetically similar at different locations and depths. However, they were separated from terrestrial myxobacteria at high levels of classification. This discovery indicates that the marine myxobacteria are phylogeographically separated from their terrestrial relatives, likely because of geographic separation and environment selection.
Similar content being viewed by others
Introduction
A long-held concept in microbial ecology is that microorganisms are globally dispersed and are able to proliferate in any habitat that supports their lifestyles. Molecular surveys have revealed a great diversity of bacteria in almost all surveyed environmental samples (Giovannoni and Stingl, 2005; Lozupone and Knight, 2007). Analysis of the tremendous amount of data collected in these molecular surveys suggests that, similar to macroorganisms, free-living microorganisms exhibit biogeographical distribution patterns (Martiny et al., 2006; Green et al., 2008; Fuhrman, 2009). However, this suggestion has mainly been based on the general construction of microorganisms in different locations, and has not yet been testified in any specific microbial taxon. For a specific microbial taxon, there are two possible geographical patterns: the same subtaxa could share many environments to which all members are adapted, or different subtaxa could be found specifically within each environment. To understand microbial ecology more fully, exploring the distribution patterns of given taxonomic units in different microbial communities is required, but such studies have rarely been conducted.
Myxobacteria are phylogenetically located in the δ-division of Proteobacteria. Owing to their unique social lifestyle, the myxobacteria are of particular interest in studies of multicellular behavior in bacteria (Rosenberg, 1984; Dworkin and Kaiser, 1993; Whitworth, 2007). Myxobacterial cells exhibit social behavior during each stage of the life cycle. For example, myxobacterial cells glide on solid surfaces in swarms and feed on macromolecules and other microbial cells in groups, and most cultured myxobacterial strains develop multicellular resting structures called fruiting bodies that contain myxospores for long-time survival after food sources have been exhausted (Shimkets, 1990). Cultured myxobacteria have been identified in various terrestrial environments by inducing the formation of fruiting bodies, and thus they are regarded as typical soil microorganisms (Reichenbach, 1999; Dawid, 2000). Although oceans occupy most of our biosphere and contain a tremendous diversity of microorganisms (Whitman et al., 1998; Lozupone and Knight, 2007), only a few halophilic (Iizuka et al., 1998) or halotolerant (Li et al., 2002) strains of myxobacteria have been isolated from coastal areas. Studies by Iizuka et al. (1998) found that myxobacteria-like swarms appeared in only 6 of 90 analyzed coastal samples, and these halophilic strains were subsequently identified to be new myxobacterial taxa (Fudou et al., 2002; Iizuka et al., 2003a, 2003b). The apparent frequency of myxobacteria in coastal samples is much lower than that in soil samples (Reichenbach, 1999; Dawid, 2000). Although no myxobacterial cultures have been obtained besides the isolates from the coasts, some of the general molecular surveys on the constructions of marine bacterial communities have identified a few myxobacteria-related 16S rRNA gene sequences in deep-sea or hydrothermal vent sediments (Moyer et al., 1995; Ravenschlag et al., 1999; Pham et al., 2008). Furthermore, recent high-throughput 454 pyrosequencing has also revealed some short myxobacteria-related fragments of the hypervariable region sequences of the 16S rRNA gene in different marine samples (Galand et al., 2009; Gilbert et al., 2009; Andersson et al., 2010). However, until now, the existence and distribution of myxobacteria in the ocean have not been thoroughly investigated. It is unknown whether myxobacteria are common in oceanic environments, whether the marine myxobacteria discovered in coastal samples are indigenous to the ocean or whether they have adapted from soil populations. In this study, we sought to explore the prevalence of myxobacteria in the marine sediments and to investigate their phylogenetic relationships with their terrestrial counterparts.
Owing to their unique lifestyles and characteristics, myxobacteria are in low abundance in natural environments, as estimated by culturing (Reichenbach, 1999; Dawid, 2000) and molecular methods (Wu et al., 2005). To explore the existence of myxobacteria, we need to develop specific methods to retrieve them from the total bacterial population. In our previous work (Wu et al., 2005; Jiang et al., 2007), in addition to culturing methods, we assessed the existence of myxobacteria in a soil niche by constructing myxobacteria-enriched libraries of 16S rRNA gene sequences using semispecific primer pairs. In this study, we used similar specific methods to explore the population of myxobacteria in marine sediments. Although almost no myxobacteria were obtained by culturing methods, molecular surveys revealed many myxobacteria-related 16S rRNA gene sequences in five sediments from deep-sea and hydrothermal vents. Interestingly, the marine myxobacterial 16S rRNA gene sequences (including those sequences from other marine samples retrieved from GenBank) were phylogenetically similar at different depths and locations, but they were normally separate from the soil myxobacteria at high levels of classification, indicating a phylogeographical separation between myxobacteria in the ocean and on land. Our discoveries provide new insights into the phylogeny of this unique bacterial group and their existence, distribution and evolution in nature.
Materials and methods
Sediment samples
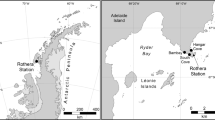
The five marine sediments were sampled at different locations and depths by means of the remotely operative vehicle of Japan Agency for Marine-Earth Science and Technology (JAMSTEC) during 2004 and 2006. After collection, the samples were freeze-dried and stored at −20 °C. Detailed information of these samples is summarized in Table 1.
Isolation techniques
Marine myxobacteria in the sediments were enriched and isolated using previously described techniques (Li et al., 2002). Briefly, the enrichment media were prepared in different concentrations of seawater, and colonies with the characteristics of myxobacteria-type swarms or those with fruiting body-like structures were transferred to new media for further identification.
Construction of 16S rRNA gene libraries
Total DNA was extracted from the marine sediments using the UltraClean Soil DNA Kit Mega Prep (MO BIO Laboratories, Carlsbad, CA, USA) following the manufacturer's instructions. Three primer sets, 27F/1492R, W1/1492R and W4/1492R, were used to amplify the 16S rRNA gene sequences from the extracted DNA as described in our previous studies (Wu et al., 2005; Jiang et al., 2007). W1 and W4 were designed on the basis of the 16S rRNA gene sequences of cultured strains of Cystobacterineae and Sorangineae, respectively. Primers 27F and 1492R are universal primers for bacteria. It is noteworthy that the genera of Nannocystis and Kofleria flava (previously named Polyangium vitellinum) in the present suborder Nannocystineae (Reichenbach, 2004; Shimkets et al., 2005) had been placed in the former Sorangineae suborder (Reichenbach and Dworkin, 1992). The myxobacteria-specific primer W4 is consistent with the currently available cultured myxobacteria of Sorangineae and Nannocystineae, including the recently discovered marine halophilic myxobacteria (Iizuka et al., 1998, 2003a, 2003b; Fudou et al., 2002) that were later included in the new suborder Nannocystineae (Reichenbach, 2004; Shimkets et al., 2005). Primer W1 also corresponds to the more recently discovered Anaeromyxobacter species (Sanford et al., 2002), which is located in the suborder Cystobacterineae. Thus, W1 is specific to the suborder Cystobacterineae, whereas W4 corresponds to both Sorangineae and Nannocystineae according to the present taxonomy of myxobacteria. A touchdown PCR protocol was used to increase the diversity of amplification products (Wu et al., 2005). Amplified products were purified using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA, USA). They were then ligated into the pGEM-T easy Vector (Promega, Madison, WI, USA) following the protocols recommended by the manufacturer, and transferred into the Escherichia coli strain JM109 by electroporation. For each of the five marine samples, three libraries of 16S rRNA gene sequences were thus established: the universal bacterial library (from the amplification products with the primer pair 27F/1492R), the Cystobacterineae-enriched library (from W1/1492R) and the Sorangineae/Nannocystineae-enriched library (from W4/1492R).
In situ colony hybridization and sequencing
In situ colony hybridization was carried out following our previously established protocols (Jiang et al., 2007). Briefly, the Cystobacterineae-specific oligonucleotide W2 (5′-GTAAAGCACTTTCGACCG-3′; corresponding to base pairs 427−444 of 16S rRNA in E. coli) and the Sorangineae-specific oligonucleotide W5 (5′-GTAAGACAGAGGGTGCAAACGT-3′; corresponding to base pairs 529−550) were used as probes to hybridize the clones in the W1/1492R and W4/1492R libraries. W5 is specific to Sorangineae rather than Nannocystineae according to the present taxonomy of myxobacteria (Jiang et al., 2007). A Nannocystineae probe was not designed because of the limited number of 16S rRNA gene sequences available for this suborder. The probes were labeled at the 3′ end with fluorescein-2′-deoxyuridine, 5′-triphosphate by terminal transferase using the Gene Image 3′-oligo labeling module (Amersham Biosciences, Piscataway, NJ, USA) in accordance to the manufacturer's instruction. The Gene Image CDP-Star detection module (Amersham Biosciences) was used to detect the hybridization signal according to the manufacturer's instructions. Sequencing was carried out from two directions at the Shanghai Sangon Sequencing Center (Shanghai, China).
Phylogenetic analysis
The 16S rRNA gene sequences were first screened using the Naïve Bayesian Classifier of Ribosomal Database Project II (Wang et al., 2007b) to eliminate nonmyxobacterial sequences. The relationships between different marine myxobacteria-related sequences were analyzed in an unrooted tree constructed using the interior branch test of the neighbor-joining method in MEGA version 3.1 (Kumar et al., 2004). For comprehensive phylogenetic analyses, all the myxobacteria-related 16S rRNA gene sequences were retrieved from the GenBank databases using the searching item: Myxococcales AND 16S. The myxobacterial sequences from different sources were then aligned in the ClustalX (1.83) program (Thompson et al., 1997). The same sequence sections (1172 bp length after the alignment) were selected for the construction of a phylogenetic tree in the MEGA program (Kumar et al., 2004). To produce a concise tree, we removed redundant sequences (from same samples and with a similarity of 98% or higher) by hand after primary analyses. The 16S rRNA gene sequence of the δ-proteobacterium Desulfovibrio desulfuricans (GenBank accession no. M34113) was used as an outgroup to root the tree. Bootstrapping supports for the construction of phylogenetic trees were calculated from a sample of 1000 replicates.
The short 16S rRNA gene sequences that were excluded from the construction of the phylogenetic tree were further searched of their highly similar sequences using the BLAST program against the data set of the tree members to evaluate their phylogenetic positions in the tree. The hypervariable region V6 sequences of the 16S rRNA gene from marine samples, which were produced by high-throughput 454 pyrosequencing, were retrieved from corresponding databases and screened myxobacteria-related sequences using the Naive Bayesian Classifier of Ribosomal Database Project II (Wang et al., 2007b). These short myxobacterial V6 region fragments also evaluated their positions in the phylogenetic tree using the BLAST program.
Sequence data
The sequence data have been submitted to the GenBank database. The data accession numbers in GenBank are EU437468 to EU437535 and EU438124 to EU438749 for the 16S rRNA gene sequences of uncultured marine myxobacteria and nonmyxobacteria, respectively. FJ418075 to FJ418127 are the newly obtained 16S rRNA gene sequences of myxobacteria from a campus soil Sorangineae/Nannocystineae-enriched library (Jiang et al., 2007).
Results
Myxobacteria exist in marine sediments at different locations and depths
The marine samples analyzed in this paper were collected from either microbial mats or the surfaces of core sediments at different depths (from 204 to 4675 m) and different locations (including a hydrothermal vent system in Kagoshima Bay) (Table 1). To assess the existence and abundance of myxobacteria in marine bacterial communities, we established universal libraries of bacterial 16S rRNA gene sequences of two deep-sea sediments at depths of 853 and 4675 m. Random sequencing of the library clones revealed two myxobacterial sequences (of 88) in the 853 m sediments, but there were no myxobacteria-related sequences among 102 sequenced clones from the 4675 m mud (Table 2). In addition, we also tried to isolate myxobacterial strains from these samples using the previously described techniques (Li et al., 2002). Similar to our previous results from studies of coastal samples (Li et al., 2002), no myxobacteria-like colonies were found, with the exception of a halotolerant Myxococcus strain that appeared on the enriching medium of 853 m mud (detailed data not shown).
We then tried to establish myxobacteria-enriched libraries of 16S rRNA gene sequences from the five marine sediments. The primer sets were semispecific for the amplification of myxobacteria: one primer of the pair was myxobacteria-specific and designed on the basis of cultured myxobacteria, whereas the other was a universal primer for bacteria. In our previous studies on uncultured soil myxobacteria, clones in the myxobacteria-enriched libraries were screened for sequencing by a further step of colony hybridization with the Cystobacterineae probe W2 (Wu et al., 2005) or Sorangineae probe W5 (Jiang et al., 2007). After two rounds of screening, more than 90% of sequenced positive clones were myxobacteria related. In this study, we also carried out colony hybridizations on the marine myxobacteria-enriched libraries with the same probes, and this produced almost no positive signals. However, random sequencing of the myxobacteria-enriched library clones revealed many myxobacteria-related sequences in the marine samples, even in the core sediments at a depth of 4675 m (Table 3). A comparison of the marine myxobacterial sequences and W2 or W5 sequences revealed significant differences, which may account for the negative results of hybridization.
The amplification efficiencies of myxobacteria-related sequences with the semispecific primers were different in these five marine samples. In the sediments collected at moderate depths (853 and 1453 m mud) or from the hydrothermal vent at a depth of 204 m, the apparent frequency of myxobacteria was rather high (accounting for 14.6–23.2% of species). In contrast, there were only 3 myxobacterial sequences of 108 sequenced clones retrieved from the sediments at depth of 2961 m and 2 of 91 sequenced clones in the sediments collected at a depth of 4675 m. It has to note that, because of the amplification limitation and on the basis of the primers, the construction of myxobacterial sequences obtained from the myxobacteria-enriched libraries does not reflect the complete construction of marine myxobacteria in nature. However, we can conclude from the results that, similar to soil, myxobacteria are also ubiquitous in oceanic sediments.
Diversity of myxobacteria in oceanic sediments
In total, 69 myxobacteria-related 16S rRNA gene sequences (66 unique sequences and 3 repeats) were obtained from 543 randomly sequenced clones of the five libraries. These unique marine myxobacterial 16S rRNA gene sequences were highly diverse and could be sorted into 13 clades by a phylogenetic distance of approximately 10% (Figure 1; the tree also included the two myxobacterial sequences from the random sequencing of the universal library of the 853 m sediments). Most of these 68 sequences appeared in Group 1 (34 of 68; 50%) and Group 13 (20 of 68; 29.4%). Other clades were majorly composed of a single or a few sequences (G2–G12). Interestingly, the sequences from different depths (from 204 to 4675 m) and locations (including the hydrothermal vents) were promiscuously neighbored in different clades. The two and two of the three myxobacterial sequences that were obtained from the 4675 and 2961 m sediment samples (Table 3), were also located in these two groups (G1 and G13 in Figure 1). All the five sequences from the 4675 and 2961 m sediment samples were in a phylogenetic distance of <5% to other sequences. If we defined the 16S rRNA gene sequences with 95% similarity or higher as a taxonomic unit (a genus) of myxobacteria, it seems that many marine myxobacterial units were probably distributed in a cosmopolitan manner in different locations and depths in oceanic sediments. The clades containing a single or a few sequences suggest their low abundance or endemic distribution in the marine samples.
Phylogenetic sorting of the myxobacteria-related 16S rRNA gene sequences in the five marine sediments in an unrooted tree. One unit of the bar is equivalent to one nucleotide change per 100 bp. The bootstrap support is from 1000 replicates. The groups are separated by a phylogenetic distance of approximately 10%. The sequences from ‘Cbk’ and ‘367’ are marked by (*) and (**), respectively, for easy tracking.
Phylogeographical distributions of myxobacteria in the ocean and on land
To investigate the phylogenetic relationships between marine and terrestrial myxobacteria, we constructed a comprehensive phylogenetic tree using the available terrestrial and marine cultured and uncultured myxobacterial 16S rRNA gene sequences (Figure 2). For parallel analysis, clones in the preserved soil Sorangineae/Nannocystineae-enriched library were randomly sequenced to retrieve the myxobacterial sequences that were likely lost in the hybridization step in our previous studies (Jiang et al., 2007). These newly obtained soil uncultured sequences (Supplementary Table 1) were added into the construction. There were a few uncultured myxobacterial sequences in previously reported general molecular surveys of different marine samples (Moyer et al., 1995; Ravenschlag et al., 1999; Bowman et al., 2000; Arakawa et al., 2006; Pham et al., 2008; Zhang et al., 2008). Besides, some unpublished myxobacteria-related 16S rRNA gene sequences were also retrieved from the GenBank database using the searching items: Myxococcales AND 16S. These sequences were also included in the construction, if they were long enough after alignment. Because too many sequences were used for the construction, the sequences that were from same samples and with a similarity of approximately 98% or higher were removed to produce a concise phylogenetic tree of myxobacteria (Figure 2). The detailed phylogenetic tree is provided as Supplementary Figure 1.
Comprehensive phylogenetic analyses of myxobacteria using the available terrestrial and marine cultured and uncultured myxobacterial 16S rRNA gene sequences. This is a concise phylogenetic tree of myxobacteria produced by removing the sequences that were from the same samples and in approximately 98% similarity or higher. The detailed phylogenetic tree is provided as Supplementary Figure 1. The tree structure was constructed in a circle form using MEGA 3.1 program. One unit of the bar is equivalent to one nucleotide change per 100 bp. The outmost cycle was added after the construction. The bootstrap support is from 1000 replicates. The phylogenetic clades in the tree are separated by a phylogenetic distance of approximately 10%. The uncultured marine myxobacterial sequences that were retrieved from previous reports are underlined for tracking. It is noteworthy that the marine myxobacterium strain NU-2, which was isolated from a salt soil sample collected from the coast of the Huanghai Sea, China, is far distant from any other cultured myxobacteria, although it was designated Nannocystis sp. (Zhang et al., 2002).
In the present taxonomy of the Myxococcales order, there are three suborders, Cystobacterineae, Sorangineae and Nannocystineae (Reichenbach, 2004; Shimkets et al., 2005). However, inferred from the comprehensive tree of the available cultured and uncultured, myxobacteria can be divided into much more clades by a phylogenetic distance of approximately 10% or longer (41 clades in Figure 2). The suborder Cystobacterineae (located in C1) was composed of many cultured and uncultured terrestrial sequences. Similarly, all the discovered cultured and uncultured members of the Sorangineae suborder (C6) were from terrestrial environments. However, in the current Nannocystineae suborder (C26–C37), the marine and terrestrial sequences were rather miscellaneously distributed. Most of the available marine cultured and uncultured myxobacterial sequences were located in this big group. There are two identified terrestrial genera in the suborder Nannocystineae, Kofleria and Nannocystis (Reichenbach, 2004). However, these two genera are separated by a phylogenetic distance of approximately 15% (Spröer et al., 1999). The cultured halophilic marine myxobacteria that were isolated from coastal environments (Fudou et al., 2002; Iizuka et al., 2003a, 2003b) were either neighbored with Kofleria at a phylogenetic distance of 4% (Haliangium in C37) or clustered by themselves (Plesiocystis and Enhygromyxa in C26). Nannocystis formed a single clade, closely neighbored with an uncultured soil sequence (C27). The marine myxobacterial sequences in Nannocystineae suborder were all in low similarity to the Nannocystis subgroup (with a phylogenetic distance of approximately 10% or longer). In contrast, the Kofleria subgroup (C37) was composed of uncultured marine myxobacterial sequences and cultured marine species from different depths and locations at a phylogenetic distance of <8%, with the exception of Kofleria. The uncultured members of the C37 clade were not only from the five marine sediments, but also from eastern Antarctica (AF142837∣BURTON-17, see Bowman et al., 2000), and permanently cold marine sediments (AJ297457∣Sva0767b and AJ241020∣Sva0679, Ravenschlag et al., 1999). This suggests that in the three documented suborders, the newly established Nannocystineae is a mixture of marine and terrestrial myxobacteria, and should be divided into two or more suborders. The Kofleria subgroup is suggested to be a marine clade, whereas the Nannocystis subgroup is a terrestrial clade.
The uncultured sequences not only neighbored with the cultured myxobacteria, but majorly clustered into many unknown taxonomic clades. Interestingly, these unknown taxonomic clades were also almost completely composed of either soil or marine uncultured sequences. The marine sequences from different locations and depths of the ocean were normally themselves grouped into clades, and had a long phylogenetic distance from the terrestrial sequences (more than 10%). For example, the members of the C13 clade included the sequences from our five marine sediments, as well as the sequences from bacterial communities associated with the Caribbean coral Montastrea faveola (FJ425609∣MD3.21) and anoxic sediments from the Marmara Sea (AM991014∣Iz17_12). Thus, the marine myxobacteria are mostly indigenous. They are phylogeographically separated from their terrestrial counterparts at high levels of classification.
However, it is clear to see that there are some exceptions of the above general geographic patterns of myxobacteria. A few marine and terrestrial myxobacterial sequences were found in the opposing environment. For example, D030-W4-42∣EU437515 is a sequence from the 1453 m sediment sample, but is located in a terrestrial clade (C7) with a phylogenetic distance of approximate 8% to their soil counterparts. AY711420∣SIMO-2054 is from salt marsh, but neighbors with terrestrial uncultured sequences at a phylogenetic distance of <4% (in C8). The uncultured soil sequence 79-49∣AY803794 is clustered into the C28 clade and neighbors with the uncultured marine myxobacterial sequences from different oceanic locations at a phylogenetic distance of approximately 8%. Furthermore, Kofleria is a cultured soil genus, but closely neighbors with marine myxobacteria in the Kofleria subgroup C37. A halotolerant Myxococcus strain appeared on the enriching medium of 853 m mud when we tried to culture marine myxobacteria from the marine sediments. We note that the marine myxobacteria-enriched libraries in this study were established with the same primers for the construction of the terrestrial myxobacteria-enriched libraries (Wu et al., 2005; Jiang et al., 2007), but a few sequences that are phylogenetically close to the terrestrial myxobacteria are present in the deep-sea and hydrothermal vent sediments. The misplaced myxobacteria are thus probably the result of migrations and acclimations of soil or marine strains to the opposing conditions. Such adaptation strains are present, but are suggested to account for a small part of the marine myxobacterial communities in deep-sea and hydrothermal vent sediments.
After the above phylogenetic analysis, the short marine myxobacterial sequences that were excluded from the tree construction were phylogenetically analyzed using the BLAST program. These short sequences still followed the above phylogeographical distribution patterns, and located in marine clades (the analytic results are provided in Supplementary Table 2). In addition, the high-throughput 454 pyrosequencing has recently been used to quantitatively describe the construction of microbial communities (von Mering et al., 2007). Normally, the hypervariable regions, such as V6, of the 16S rRNA gene were analyzed. The marine short V6 region sequences (the available data sets were all from seawater samples) were retrieved from corresponding databases, and screened myxobacteria-related sequences using the Naïve Bayesian Classifier of Ribosomal Database Project II (Wang et al., 2007b). It is interesting to see that there are a few marine myxobacteria-related sequences in seawater samples. For example, only one myxobacteria-related sequence was retrieved from a total of 182 560 V6 region sequences in seawater samples of the western English Channel (Gilbert et al., 2009). In deep water masses of the rare biosphere in the Arctic Ocean, only 20 of the 740 353 V6 hypervariable region sequences were myxobacteria-related (Galand et al., 2009). However, the 16S rRNA gene fragments produced by 454 sequencing are too short (approximately 100 bp length) to be used in accurate phylogenetic analyses. These short sequences may be highly similar to several sequences that are located in different clades, but normally closer to marine myxobacterial sequences. Interestingly, if a marine area was frequently influenced by freshwater, many terrestrial myxobacterial sequences would appear in the marine bacterial communities (Andersson et al., 2010), which also indicates that the marine and terrestrial myxobacteria are phylogeographically separate. The above analytic results are summarized in Supplementary Table 2.
Discussion
Two central questions of microbial biogeography are whether microbial assemblages differ in different locations and, if so, what causes the spatial variation (Martiny et al., 2006). Ocean and soil are the two largest distinct habitats for living organisms on Earth. Usually, similar low taxonomic units (genus or species) of free-living bacteria can be found in both terrestrial and oceanic environments, but with distinct characteristics resulting from adaptation to each environment. For example, although Salinispora is an obligate marine genus (Maldonado et al., 2005), many genera or species of Actinomycetes were cultured from either oceanic or soil samples. It is reasonably suggested that myxobacteria are able to exist in oceanic conditions. If myxobacteria are present in marine sediments, they might be indigenous, they might have originated from soil and adapted to the marine conditions or they could simply be in a resting stage. Studies of few halotolerant myxobacterial cultures have suggested that soil myxobacterial strains cannot survive for long in a resting type in deep-sea or hydrothermal vent sediments. However, although similar fruiting myxobacteria taxa have been frequently isolated from different soils throughout the world, this paper discovered that phylogenetically separated 16S rRNA gene sequences of uncultured marine myxobacteria were ubiquitous and rather consistent in different places and depths of the ocean. We suggest that the present isolation protocols for myxobacteria do not enrich the marine strains. It is also possible that the marine strains do not exhibit or possess the known characteristics typical of colonies of cultured myxobacterial strains. Phylogenetic branching of marine and terrestrial myxobacteria at high levels of classification not only supports the hypothesis of microbial biogeography (Martiny et al., 2006; Green et al., 2008), but also suggests distinct and strict requirements of the lifestyles of marine and soil myxobacteria.
High concentrations of salinity in the oceanic environment might be one key factor that influences the phylogeographical separation of myxobacteria and could result in shifts of living patterns of the marine myxobacteria in response to salinity. For example, cultured marine halotolerant and halophilic myxobacterial strains isolated from coastal areas are able to develop fruiting body structures (Zhang et al., 2005). The halophilic myxobacterial strains develop fruiting body-like structures in the presence of seawater; decreasing the concentrations of seawater limits the morphogenetic process. In contrast, although the terrestrial myxobacterial isolates are usually unable to tolerate high concentrations of salinity (Reichenbach, 1999; Li et al., 2002), the halotolerant Myxococcus strains, which are suggested to be the adaptations of soil Myxococcus strains to the ocean (Zhang et al., 2005), form fruiting bodies only in the presence of diluted seawater or in the absence of seawater. Increasing the concentration of seawater, decreases the morphogenetic capabilities of these halotolerants (Zhang et al., 2005; Wang et al., 2007a). High concentrations of salt in the ocean thus inhibit the formation of fruiting body structures in terrestrial myxobacteria, but may be required for the morphogenetic process of marine myxobacteria. Differences between marine and terrestrial environments have greatly limited the cross migration of myxobacteria. In geographically separated environments, indigenous myxobacteria evolved separately in the ocean and on land, forming the present divaricated phylogenetic clades of marine and terrestrial myxobacteria.
Myxobacteria are characterized among the prokaryotes by their unique social behavior, which is also a limiting factor for ecological studies of these species. Owing to the difficulties of cultivation, there are no myxobacterial isolates from deep-sea or hydrothermal vents. After determining the ubiquity of myxobacteria in nature, many questions still remain. The lifestyles and characteristics of the marine myxobacteria are unknown. For example, it would be interesting to determine whether the myxobacteria undergo a similar morphogenetic process to form complicated multicellular structures, and whether they function as microbial predators in the ocean, similar to soil myxobacteria. Molecular surveys may not only reveal the phylogeny of marine myxobacteria, as well as information related to their existence and distribution, but also provide a guide to the development of isolation techniques. Cultured myxobacteria are highly promising in the production of many kinds of bioactive compounds (Reichenbach, 2001; Gerth et al., 2003). Understanding the involving mechanisms by halotolerant or halophilic myxobacterial strains to marine conditions is beneficial for the exploration of marine myxobacterial resources (Zhang et al., 2005, 2007; Wang et al., 2007a; Pan et al., 2009). The marine myxobacteria may provide new sources of myxobacteria for drug screening. Our improved understanding and use of myxobacteria will require innovative studies that develop new isolation techniques independent of fruiting body formation as well as metagenome sequencing projects on the whole microbial community in a niche.
References
Andersson AF, Riemann L, Bertilsson S . (2010). Pyrosequencing reveals contrasting seasonal dynamics of taxa within Baltic Sea bacterioplankton communities. ISME J 4: 171–181.
Arakawa S, Sato T, Sato R, Zhang J, Gamo T, Tsunogai U et al. (2006). Molecular phylogenetic and chemical analyses of the microbial mats in deep-sea cold seep sediments at the northeastern Japan Sea. Extremophiles 10: 311–319.
Bowman JP, Rea SM, McCammon SA, McMeekin TA . (2000). Diversity and community structure within anoxic sediment from marine salinity meromictic lakes and a coastal meromictic marine basin, Vestfold Hilds, Eastern Antarctica. Environ Microbiol 2: 227–237.
Dawid W . (2000). Biology and global distribution of myxobacteria in soils. FEMS Microbiol Rev 24: 403–427.
Dworkin M, Kaiser D . (1993). Myxobacteria II. ASM Press: Washington, DC.
Fudou R, Jojima Y, Iizuka T, Yamanaka S . (2002). Haliangium ochraceum gen. nov., sp. nov. and Haliangium tepidum sp. nov.: novel moderately halophilic myxobacteria isolated from coastal saline environments. J Gen Appl Microbiol 48: 109–116.
Fuhrman JA . (2009). Microbial community structure and its functional implications. Nature 459: 193–199.
Galand PE, Casamayor EO, Kirchman DL, Lovejoy C . (2009). Ecology of the rare microbial biosphere of the Arctic Ocean. Proc Natl Acad Sci USA 106: 22427–22432.
Gerth K, Pradella S, Perlova O, Beyer S, Müller R . (2003). Myxobacteria: proficient producers of novel natural products with various biological activities—past and future biotechnological aspects with the focus on the genus Sorangium. J Biotechnol 106: 233–253.
Gilbert JA, Field D, Swift P, Newbold L, Oliver A, Smyth T et al. (2009). The seasonal structure of microbial communities in the Western English Channel. Environ Microbiol 11: 3132–3139.
Giovannoni SJ, Stingl U . (2005). Molecular diversity and ecology of microbial plankton. Nature 437: 343–348.
Green JL, Bohannan BJM, Whitaker RJ . (2008). Microbial biogeography: from taxonomy to traits. Science 320: 1039–1043.
Iizuka T, Jojima Y, Fudou R, Hiraishi A, Ahn JW, Yamanaka S . (2003a). Plesiocystis pacifica gen. nov., sp. nov., a marine myxobacterium that contains dihydrogenated menaquinone, isolated from the Pacific coasts of Japan. Int J Syst Evol Microbiol 53: 189–195.
Iizuka T, Jojima Y, Fudou R, Tokura M, Hiraishi A, Yamanaka S . (2003b). Enhygromyxa salina gen. nov., sp. nov., a slightly halophilic myxobacterium isolated from the coastal areas of Japan. Syst Appl Microbiol 26: 189–196.
Iizuka T, Jojima Y, Fudou R, Yamanaka S . (1998). Isolation of myxobacteria from the marine environment. FEMS Microbiol Lett 169: 317–322.
Jiang DM, Wu ZH, Zhao JY, Li YZ . (2007). Fruiting and non-fruiting myxobacteria: a phylogenetic perspective of cultured and uncultured members of this group. Mol Phylogenet Evol 44: 545–552.
Kumar S, Tamura K, Nei M . (2004). MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings Bioinformat 5: 150–163.
Li YZ, Hu W, Zhang YQ, Qiu ZJ, Zhang Y, Wu BH . (2002). A simple method to isolate salt-tolerant myxobacteria from marine samples. J Microbiol Methods 50: 205–209.
Lozupone CA, Knight R . (2007). Global patterns in bacterial diversity. Proc Natl Acad Sci USA 104: 11436–11440.
Maldonado LA, Fenical W, Jensen PR, Kauffman CA, Mincer TJ, Ward AC et al. (2005). Salinispora arenicola gen. nov., sp. nov. and Salinispora tropica sp. nov., obligate marine actinomycetes belonging to the family Micromonosporaceae. Int J Syst Evol Microbiol 55: 1759–1766.
Martiny JB, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL et al. (2006). Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4: 102–112.
Moyer CL, Dobbs FC, Karl DM . (1995). Phylogenetic diversity of the bacterial community from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol 61: 1555–1562.
Pan HW, Liu H, Liu T, Li CY, Li ZF, Cai K et al. (2009). Seawater-regulated genes for two-component systems and outer membrane proteins in Myxococcus. J Bacteriol 191: 2102–2111.
Pham VD, Konstantinidis KT, Palden T, DeLong EF . (2008). Phylogenetic analyses of ribosomal DNA-containing bacterioplankton genome fragments from a 4000 m vertical profile in the North Pacific Subtropical Gyre. Environ Microbiol 10: 2313–2330.
Ravenschlag K, Sahm K, Pernthaler J, Amann R . (1999). High bacterial diversity in permanently cold marine sediments. Appl Environ Microbiol 65: 3982–3989.
Reichenbach H . (1999). The ecology of the myxobacteria. Environ Microbiol 1: 15–21.
Reichenbach H . (2001). Myxobacteria, producers of novel bioactive substances. J Ind Microbiol Biotechnol 27: 149–156.
Reichenbach H . (2004). The Myxococcales. Bergey's Manual of Systematic Bacteriology, Garrity GM (ed). Springer-Verlag: New York, NY, pp 1059–1143.
Reichenbach H, Dworkin M . (1992). The myxobacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds). The Prokaryotes. 2nd ed. Springer-Verlag: New York, NY, pp 3416–3487.
Rosenberg E . (1984). Myxobacteria: Development and Cell Interactions. Springer-Verlag: New York, NY.
Sanford RA, Cole JR, Tiedje JM . (2002). Characterization and description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an aryl-halorespiring facultative anaerobic myxobacterium. Appl Environ Microbiol 68: 893–900.
Shimkets LJ . (1990). Social and developmental biology of myxobacteria. Microbiol Rev 54: 473–501.
Shimkets LJ, Dworkin M, Reichenbach H . (2005). The myxobacteria. The Prokaryotes 3rd edn (release 3.19) Springer-Verlag: New York, NY.
Spröer C, Reichenbach H, Stackebrandt E . (1999). The correlation between morphogenetic classification of myxobacteria. Int J Syst Bacteriol 49: 1255–1262.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG . (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882.
von Mering C, Hugenholtz P, Raes J, Tringe SG, Doerks T, Jensen LJ et al. (2007). Quantitative phylogenetic assessment of microbial communities in diverse environments. Science 315: 1126–1130.
Wang B, Hu W, Liu H, Zhang CY, Zhao JY, Jiang DM et al. (2007a). Adaptation of salt-tolerant Myxococcus strains and their motility systems to the ocean conditions. Microb Ecol 54: 43–51.
Wang Q, Garrity GM, Tiedje JM, Cole JR . (2007b). Naive Bayesian Classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261–5267.
Whitman WB, Coleman DC, Wiebe WJ . (1998). Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95: 6578–6583.
Whitworth DE . (2007). Myxobacteria: Multicellularity and Differentiation. ASM Press: Washington, DC.
Wu ZH, Jiang DM, Li P, Li YZ . (2005). Exploring the diversity of myxobacteria in a soil niche by myxobacteria-specific primers and probes. Environ Microbiol 7: 1602–1610.
Zhang CY, Cai K, Liu H, Zhang Y, Pan HW, Wang B et al. (2007). A new locus important for Myxococcus social motility and development. J Bacteriol 189: 7937–7941.
Zhang J, Liu Z, Wang S, Jiang P . (2002). Characterization of a bioflocculant produced by the marine myxobacterium Nannocystis sp. NU-2. Appl Microbiol Biotechnol 59: 517–522.
Zhang W, Ki J-S, Qian P-Y . (2008). Microbial diversity in polluted harbor sediments I: bacterial community assessment based on four clone libraries of 16S rDNA. Estuar Coast Shelf Sci 76: 668–681.
Zhang YQ, Li YZ, Wang B, Wu ZZ, Zhang CZ, Gong X et al. (2005). Characteristics and living patterns of marine myxobacterial isolates. Appl Environ Microbiol 71: 3331–3336.
Acknowledgements
This work was financially supported by National Science Foundation for Distinguished Young Scholars (No. 30825001), National Natural Science Foundation (No. 30671192 and 30870001) and National High Technology Research and Development program (No. 2007AA021501) of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Jiang, DM., Kato, C., Zhou, XW. et al. Phylogeographic separation of marine and soil myxobacteria at high levels of classification. ISME J 4, 1520–1530 (2010). https://doi.org/10.1038/ismej.2010.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2010.84
Keywords
This article is cited by
-
Bradymonabacteria, a novel bacterial predator group with versatile survival strategies in saline environments
Microbiome (2020)
-
Compositional homogeneity in the pathobiome of a new, slow-spreading coral disease
Microbiome (2019)
-
Analysis of the Genome and Metabolome of Marine Myxobacteria Reveals High Potential for Biosynthesis of Novel Specialized Metabolites
Scientific Reports (2018)
-
Current trends in myxobacteria research
Annals of Microbiology (2016)
-
Metagenomic analysis of size-fractionated picoplankton in a marine oxygen minimum zone
The ISME Journal (2014)