Abstract
Plankton represent a nutrient-rich reservoir capable of enriching Vibrio species, which can include human pathogens, at higher densities than the surrounding water column. To better understand the relationship between vibrios and plankton, the partitioning of culturable vibrios, on TCBS, between free living and plankton associated (63–200- and >200-μm-size fractions) was monitored over a 1-year period in coastal waters of Georgia, USA. Seasonal changes in the total Vibrio concentration were then compared with changes in environmental parameters as well as changes in the relative composition of the plankton community. Using univariate analyses, Vibrio concentrations were strongly associated with temperature, especially when those vibrios were plankton associated (R2=0.69 and 0.88 for the water and both plankton fractions; respectively) (P<0.01). Multivariate general linear models revealed that Vibrio concentrations in the plankton fractions were also correlated to shifts in the relative abundance of specific plankton taxa. In the 63–200-μm fraction, Vibrio concentrations were inversely associated with copepods, cyanobacteria and diatoms. In the >200-μm fraction, Vibrio concentrations were positively associated with copepods and negatively associated with decapod larvae. Our results confirm the role of temperature in Vibrio seasonality and highlight an important and independent role for plankton composition in explaining seasonal changes in Vibrio concentration.
Similar content being viewed by others
Introduction
Vibrio species are autochthonous members of the bacterial community in warm estuarine and coastal waters worldwide (Thompson et al., 2003a). They are among the most abundant bacteria readily cultured from the marine environment and exhibit distinct seasonal variation in population density and diversity (Thompson et al., 2003a). Ecologically, vibrios play an important role in the degradation of organic matter and act as a link that transfers dissolved organic carbon to higher trophic levels of the marine food web (Grossart et al., 2005). Epidemiologically, at least 12 Vibrio species are known to be important pathogens of humans and marine animals (Colwell and Grimes, 1984; Morris Jr, 2003; Panicker et al., 2004).
The association of vibrios with planktonic organisms, especially copepods, has been suggested as an important component of Vibrio ecology, especially for Vibrio cholerae (Sochard et al., 1979; Huq et al., 1983; Huq et al., 2005). Plankton represent organic-rich microenvironments (Long and Azam, 2001; Grossart et al., 2005), and the high nutrient concentrations of the plankton microhabitat can selectively enrich heterotrophic bacteria, including vibrios (Huq et al., 1983; Tamplin et al., 1990; Lipp et al., 2003; Long et al., 2005). The production of an extracellular chitinase allows vibrios to utilize the chitinous exoskeletons of some plankton taxa as a source of carbon and nitrogen (Thompson et al., 2003a), leading to the hypothesis that some vibrios may show a competitive advantage when plankton associated (Heidelberg et al., 2002a).
Plankton colonized by pathogenic Vibrio species can potentially act as a vehicle of disease transmission, as in the case of cholera (Huq and Colwell, 1996; Huq et al., 2005); however, the details of the interactions between other members of the Vibrio genus and the plankton community remain largely unknown. Recent studies indicate that the structure and composition of the heterotrophic bacterial community may be dependent upon the structure and composition of the plankton community (Riemann et al., 2000; Fandino et al., 2001; Pinhassi et al., 2004). It has also been hypothesized that individual plankton species may harbor specific bacterial communities (Grossart et al., 2005).
The objective of this study was to determine whether changes in the composition of the plankton community contribute to the seasonality of the total culturable Vibrio population, either synergistically with or independent of temperature. Several studies have described the relationship between vibrios and plankton by characterizing the Vibrio population as either free living or plankton associated (Baffone et al., 2006), but few studies have addressed the relationship between the structure and composition of the plankton community and that of the Vibrio population (Heidelberg et al., 2002a; Grossart et al., 2005; Huq et al., 2005). We hypothesized that shifts in the composition of the plankton reservoir contribute to the observed seasonality in the Vibrio population.
Materials and methods
Sampling sites
This study included 12 sampling sites along the coast of Georgia, USA (Figure 1). The study sites were representative of two distinct estuaries, Sapelo Sound and Wassaw Sound. Typical of the South Atlantic Bight (southeastern USA) and coastal Georgia in particular, mixing in these estuaries is dominated by tidal exchange (Verity et al., 2006). Furthermore, these two estuaries are unique in that they receive little direct river input.
Sampling sites were selected in coordination with an on-going water quality program administered by the Georgia Department of Natural Resources. Sites were selected to represent a range of environments and hydrological conditions including tidal creeks and open-water sounds. Each site was part of a network of oyster beds open for commercial or public harvest. The 12 stations were divided equally between Wassaw Sound (Chatham County) and Sapelo Sound (McIntosh County) and were sampled bi-monthly beginning in January 2006 and ending in February 2007.
Sample collection
Monthly sample collection coincided with the ebb tide of spring tide events. Surface temperature (°C), salinity and dissolved oxygen (DO) (% saturation) were recorded at each sampling site using a YSI Multi-meter (YSI Environmental, model 556, Yellow Springs, OH, USA). Three fractions were collected from each site: surface water and two plankton fractions (63–200 and >200 μm). Briefly, approximately 1 l of surface water was collected in a sterile polypropylene bottle. Plankton fractions were collected by a 5-min horizontal tow of 63- and 200-μm plankton nets (Sea-Gear Corp., model 9000, Melbourne, FL, USA) at <1 m depth. The 63-μm fraction was subsequently filtered through a 200-μm mesh. Plankton samples were then washed and resuspended to a volume of 1 l in a sterile polypropylene bottle using sterile phosphate-buffered saline. Sampling was completed within a 6-h period, beginning early in the morning. Samples were stored in a cooler filled with ambient seawater and transported to the lab, and were processed on the same day. Temperature variability during transit was monitored using a max/min thermometer (SPER Scientific, no. 736690, Scottsdale, AZ, USA).
Plankton identification
A 25-ml aliquot of each plankton sample was fixed (4% v/v, formalin, final concentration) and stored at 5 °C. The wet weights (g ml−1) were determined for the fixed plankton samples. Samples were then preserved for long-term storage in 70% ethanol (v/v) and shipped to EcoAnalysts Inc. (Moscow, ID, USA) for taxonomic identification. For each sample (63–200 and >200 μm), the relative abundance of phytoplankton and zooplankton was determined separately by identifying the first 100 phytoplankton and the first 100 zooplankton encountered to the lowest taxonomical unit. Owing to variability in the lowest taxonomical unit identified across all plankton (for example, some were identified to genus whereas others only to order), identified plankton were subsequently grouped into larger common categories. For phytoplankton, individuals were categorized by class (diatoms (Bacillariophyceae)) and phylum (cyanobacteria and green algae (Chlorophyta)). For zooplankton, individuals were categorized by subclass (copepods (Copepoda)), order (decapods (Decapoda) and Sessilia) and phylum (Formanifera and Rotifera).
Enumeration of culturable vibrios
Presumptive Vibrio species were enumerated on a selective medium (thio-citrate–bile–sucrose (TCBS) cholera medium, Oxoid CM0333, Basingstoke, Hampshire, UK) (Pfeffer and Oliver, 2003; Thompson et al., 2003a). Briefly, water and plankton samples were serially diluted in phosphate-buffered saline before spread plating 100 μl on duplicate TCBS plates. At least 2–3 dilutions were plated to ensure that the plates were countable. Before dilution, the plankton samples were homogenized for 1 min at 12 000 r.p.m. using a PRO homogenizer (PRO Scientific Inc., model 200, Oxford, CT, USA). Plates were incubated in the dark at 30 °C for 16–20 h and all yellow and green colonies were counted as presumptive vibrios, and reported as colony-forming units (CFUs) per ml of water and CFU per g of plankton.
Data analysis
Bacterial counts were log transformed to fit a normal distribution (Anderson–Darling statistic, α=0.10). In cases in which Vibrio levels were below the detection limit a value of zero was assigned for statistical analysis.
Pearson's correlation coefficients were calculated to evaluate relationships between the log-transformed Vibrio concentrations (CFU per ml water and CFU per g plankton) and environmental parameters. One-way analysis of variance was used to determine the significance of differences in Vibrio concentrations between fractions, sampling sites and collection months; significance was declared when P<0.05. All univariate and bivariate analyses were carried out in MINITAB version 15.0 (MINITAB Inc., State College, PA, USA).
General linear regression models (PROC GLM; SAS Institute Inc.; version 9.1, Cary, NC, USA) were developed for each fraction (water, 63–200- and >200-μm plankton) to examine relationships between the relative abundance of each plankton taxon (in the plankton fractions), environmental variables and Vibrio concentrations. All variables found to be correlated (P<0.10) in univariate analyses, and their appropriate interaction terms were initially entered into the models. Maximum likelihood estimates of each variable parameter were determined, and variables were eliminated from the model by backward elimination when P>0.05.
Results
Environmental parameters
Among the 70 sample collection events, temperature ranged from 9.3 to 30.8 °C, DO from 48.7 to 105.8% saturation and salinity from 26.3 to 32.2 (Figure 2). Temperature during transport of samples varied by ⩽2 °C, with the exception of samples collected in July 2006, which were inadvertently stored on ice. These 18 samples (six for each fraction) were removed from analysis for culturable Vibrio counts.
Temperature and DO exhibited strong seasonal trends, whereas salinity varied less and showed no seasonal pattern. DO and temperature were inversely related (r=−0.73), whereas salinity and temperature were directly related (r=0.40) (P<0.01).
Analysis of the small plankton fraction (63–200 μm)
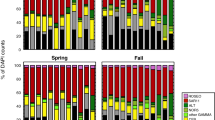
The 63–200-μm plankton samples (N=64; taxonomic identification was not available for samples collected in January 2006) were composed of phytoplankton and smaller forms of zooplankton. Diatoms (mean relative abundance 55.7%), cyanobacteria (mean relative abundance 26.1%) and green algae (mean relative abundance 17.3%) accounted for 99.1% of all phytoplankton in the 63–200-μm fractions (Figure 3). Across all phytoplankton groups, Microcystis (cyanobacteria) was the most commonly identified genus (18.6% mean relative abundance), followed by the diatoms, Skeletonema spp. and Chaetoceros spp., with mean relative abundances of 10.1 and 8.9%, respectively (Table 1).
The relative abundance of diatoms was inversely related to temperature (r=−0.40), whereas the relative abundance of cyanobacteria varied directly with temperature (r=0.41) (P<0.05 for each). No other significant associations were found.
Copepods (mean relative abundance 75.2%), Foraminifera (mean relative abundance 14.4%), Rotifera (mean relative abundance 6.4%), Sessilia (mean relative abundance 0.8%) and decapods (mean relative abundance 0.02%) accounted for 99.8% of all zooplankton in this fraction (Figure 4). Among this size fraction, most zooplankton could only be identified to the level of subclass or higher; however, among the copepods for which a finer level identification was determined, calanoid and harpacticoid were the most commonly noted at 10.9 and 3.16% relative abundance, respectively (Table 1).
The relative abundance of copepods was directly related to temperature (r=0.42), whereas the relative abundance of foraminiferida was inversely related to temperature (r=−0.42) (P<0.05 for each). No other significant associations were found.
Analysis of the large plankton fraction (>200 mm)
The >200-μm plankton samples (N=64) were composed primarily of zooplankton; however, some phytoplankton were also captured in this size fraction. Diatoms (mean relative abundance 52.2%), cyanobacteria (mean relative abundance 27.8%) and green algae (mean relative abundance 19.1%) accounted for 99.0% of all phytoplankton in this size fraction (Figure 3). Among all phytoplankton in this size fraction, Microcystis (cyanobacteria) was the most commonly noted at 16.6% mean relative abundance, followed by Thalassiosira and Oocystis at 12.7 and 9.7% mean relative abundance, respectively (Table 1). No significant correlations between the phytoplankton groups and environmental parameters were found.
Copepods (mean relative abundance 77.8%), decapods (mean relative abundance 15.7%), Sessilia (mean relative abundance 4.7%) and Foraminifera (mean relative abundance 0.3%) accounted for 99.9% of all zooplankton in this size fraction (Figure 4). Among all zooplankton in this size fraction, calanoid copepods were the most frequently identified order (74.64% relative abundance), followed by Brachyura (decapod) larvae at 10.4% mean relative abundance (Table 1).
The relative abundance of decapods and Sessilia was directly related to temperature (r=0.78 and 0.38, respectively), whereas the relative abundance of copepods was inversely related to temperature (r=−0.72) (P<0.05 for each).
Analysis for total culturable Vibrio
Of the 210 samples collected and processed, 192 were used in statistical analyses (samples collected in July 2006 were not included because of improper storage conditions). Fluctuations in mean monthly total culturable Vibrio concentrations followed a strong seasonal trend (Figure 2). Univariate analyses (Pearson's correlation coefficient) revealed that Vibrio concentrations in all fractions were directly related to temperature (r=0.69 for water and r=0.88 for both plankton fractions (P<0.01 for each)). Conversely, Vibrio concentrations were inversely related to DO in all fractions (r=−0.49, −0.67 and −0.68 for the water, 63–200- and >200-μm fractions, respectively (P<0.01 for each)). Salinity showed a positive, although weak, correlation with Vibrio concentrations (r=0.36, 0.35 and 0.39 for the water, 63–200- and >200-μm fractions, respectively (P<0.01 for each)).
The mean Vibrio concentration in the water fraction (500 CFU ml−1 (N=64)) was up to 6 orders of magnitude less than the mean concentration of vibrios in the plankton fractions (4.91 × 107 and 1.58 × 108 CFU g−1 for the 63–200- and >200-μm fractions, respectively (N=64 for both)) (Figure 2). Vibrio concentrations in the water fraction ranged from 20 CFU ml−1 in February 2007 to 6150 CFU ml−1 in August 2006. Concentrations in the 63–200-μm fractions ranged from 1.83 × 103 CFU g−1 in February 2007 to 5.98 × 108 CFU g−1 in September 2006. Concentrations in the >200-μm fraction ranged from 7.36 × 102 CFU g−1 in January 2007 to 1.58 × 108 CFU g−1 in August 2006 (Figure 2). Vibrio concentrations were not significantly different between the two plankton fractions or between the different sampling sites (analysis of variance, P<0.001).
General linear models to describe changes in total culturable Vibrio levels over time
Multivariate general linear models (GLMs) were constructed for the water fraction (Table 2) and each plankton fraction (Tables 3 and 4 for the 63–200- and >200-μm fractions, respectively). In the 63–200-μm fraction, only models based on copepods, diatoms and cyanobacteria were significantly predictive (Table 3), and in the >200-μm fraction, only models incorporating copepods and decapods were significantly predictive (Table 4).
Water fraction model
Temperature and salinity were the only significant correlates of Vibrio concentration in the water fraction (Table 2). DO and the concentration of vibrios in both plankton fractions (63–200 and >200 μm), as well as their interaction terms, were included in the model as potential drivers; however, these variables were not significant and were removed from the final model. The model estimated that each 1-°C rise in temperature corresponded to a 0.043-fold increase in the log concentration of vibrios. Meanwhile, each unit increase in salinity corresponded to a 0.049-fold increase in the log concentration of vibrios. Although the magnitude of these parameter estimates was small, temperature accounted for 33.36% of the model variance, whereas salinity accounted for 66.64% of the model variance (Table 1).
63–200-μm fraction copepod model
This model estimated that each 1% increase in the relative abundance of copepods corresponded to a 6.41-fold decrease in the log concentration of vibrios (Table 3). Copepods accounted for 16.63% of the model variation (8.11% of this variation was independent and an additional 8.52% was through interactions with DO). Each one-log increase in Vibrio concentration in the water fraction corresponded to a 3.80-fold increase in the 63–200-μm plankton fraction. The log concentration of vibrios in the water fraction accounted for 34.37% of the model variation (16.97% independently and an additional 8.74 and 8.66% from interactions with temperature and DO, respectively). Each 1-°C increase in temperature was responsible for a modest 0.28-fold increase in the log Vibrio concentration. Independently, temperature accounted for 48.90% of the variation. Although DO was retained in the final model because it was an important interaction variable, there was no significant independent relationship between plankton-associated Vibrio concentrations and DO (Table 3).
63–200-μm fraction diatom model
This model estimated that each 1% increase in the relative abundance of diatoms corresponded to a 16.19-fold decrease in log Vibrio concentration (Table 3). Diatoms accounted for 67.22% of the model variance (18.36% independently and an additional 37.18 and 11.68% because of interactions with temperature and DO, respectively). Each one-log increase in the concentration of vibrios in the water fraction corresponded to a 0.43-fold increase in log Vibrio concentration in the 63–200-μm fraction, and accounted for 8.76% of the model variance. Each 1-°C change in temperature and each 1.0% change in DO corresponded to 0.12- and 0.093-fold decreases in log Vibrio concentrations, respectively. Independently, temperature and DO accounted for 5.84 and 18.19% of the model variance, respectively (Table 3).
63–200-μm fraction cyanobacteria model
This model estimated that each 1% increase in the relative abundance of cyanobacteria corresponded to a 28.11-fold decrease in the log concentration of vibrios (Table 3). The relative abundance of cyanobacteria accounted for 14.98% of the model variance in log Vibrio concentrations in the 63–200-μm fraction (7.36% independently and an additional 7.62% through interaction with salinity). Each one-log increase in the concentration of vibrios in the water fraction corresponded to a 1.97-fold decrease in the 63–200-μm fraction and explained 9.01% of the model variation (4.36% independently and an additional 4.65% through interaction with DO). Each 1-°C change in temperature corresponded to a 0.18-fold increase in the log concentration of vibrios and explained 65.19% of the model variance. Salinity and DO also showed significant negative associations with log Vibrio concentrations in this fraction (Table 3).
>200-μm fraction copepod model
This model estimated that each 1% increase in the relative abundance of copepods corresponded to a 16.68-fold increase in the log concentration of vibrios; however, this relationship was modified by interactions between copepods and temperature and DO that were inversely related to log Vibrio concentrations (parameter estimates= −0.38 and −0.08, respectively). The relative abundance of copepods accounted for 24.79% of the model variance (12.61% independently and an additional 8.12 and 4.06% through interactions with temperature and DO, respectively). Each one-log increase in the concentration of vibrios in the water fraction corresponded to a 6.40-fold decrease in the log concentration of vibrios in the >200-μm fraction, and accounted for 40.69% of the model variance (12.59% independently and an additional 11.92 and 16.18% through interactions with temperature and salinity, respectively). Each 1-°C increase in temperature corresponded to a 0.79-fold increase in log Vibrio concentration, and accounted for 20.01% of the model variance. Each unit increase in salinity corresponded to a 0.62-fold decrease in log Vibrio concentration, and accounted for 11.29% of the model variance (Table 4).
>200-μm fraction decapod model
This model estimated that each 1% increase in the relative abundance of decapods corresponded to a 1.32-fold decrease in the log concentration of vibrios (Table 4). The relative abundance of decapods accounted for 7.65% of the model variance. Each one-log increase in the concentration of vibrios in the water fraction corresponded to a 7.27-fold decrease in the log concentration of vibrios in the >200-μm fraction, and accounted for 37.22% of the model variance (12.68% independently and an additional 10.23 and 14.31% through interactions with temperature and salinity, respectively). Each 1-°C increase in temperature corresponded to a 0.38-fold increase in log Vibrio concentration, and accounted for 46.27% of the model variance. Each unit increase in salinity corresponded to a 0.60-fold decrease in the log Vibrio concentration, and accounted for 8.85% of the model variance (Table 4).
Discussion
Most of the earlier research investigating Vibrio–plankton interactions have been focused on the relationship between V. cholerae and copepods (for example, Huq et al., 1983, 1984, 2005; Tamplin et al., 1990); however, less is known about the more general response of Vibrio populations to natural plankton communities and specifically whether plankton community composition can explain some of the seasonal variabilities in total culturable Vibrio. Results of this study show that seasonal variations in Vibrio concentrations can be modeled using changes in both environmental variables and the composition of the plankton reservoir.
Although this study relied on a culture-based approach to quantify total Vibrio abundance (which likely underestimated the total population due to non-culturable cells), the selective medium (TCBS) has been used extensively in the past to recover a large range of Vibrio spp. and provides standard approach for comparison with other studies (Massad and Oliver, 1987; Pfeffer and Oliver, 2003; Pfeffer et al., 2003). Despite the limitation in relying only on abundance of culturable Vibrio, the results presented here support earlier findings that temperature, salinity and DO are significant drivers of Vibrio seasonality (Heidelberg et al., 2002b; Lipp et al., 2003; Thompson et al., 2003b; Baffone et al., 2006), but suggest an independent role for plankton in describing the seasonal patterns. Multivariate GLMs showed that seasonal changes in the composition of the plankton community were significantly associated with changes in co-measured total culturable Vibrio concentrations.
Factors affecting Vibrio in water
Salinity is known to be correlated with some Vibrio spp., but its significance can vary across the Vibrio genus (Thompson et al., 2003a) and change depending on the range of temperature in the system (Lipp et al., 2001; Randa et al., 2004). In this study, we noted a moderate range in temperature over the year but a very small range in salinity, which may have been due to the selection of tidally dominated study sites and collections restricted to outgoing tides. Therefore, based on earlier studies (for example, Lipp et al., 2001; Louis et al., 2003), we expected that temperature would be the dominant correlate for Vibrio abundance, which was supported by the results from Pearson Product Moment Correlation (that is, univariate analysis). However, when the more robust GLMs were applied to develop predictive models for Vibrio abundance, temperature and salinity, the only significant predictors of free-living Vibrio concentrations, both showed weak parameter estimates and salinity accounted for a greater proportion of the model variance in Vibrio concentration compared with temperature. This suggests that the relationship between temperature and salinity may be more complex than that reported earlier based on studies using univariate analysis alone. It also suggests that the free-living Vibrio community may be a distinct group of bacteria, as the GLM showed no significant relationship or interaction between Vibrio levels detected in the plankton fractions.
Factors affecting Vibrio in plankton
Although temperature and salinity remained important correlates with Vibrio abundance in both plankton fractions, the independent role of the specific composition of plankton within those fractions also significantly affected the levels of Vibrio, which may indicate a specific relationship between plankton dynamics and the Vibrio population. In both fractions (63–200 and >200 μm), the relative abundance of copepods was significantly associated with the concentration of Vibrio. In particular, parameter estimates indicated a strong direct relationship between Vibrio concentration and the relative abundance of copepods in the >200-μm fraction. Copepods have been implicated as a potential vector of V. cholerae (Huq et al., 1996; Colwell et al., 2003), and an ecological relationship between V. cholerae and copepods has been suggested earlier (Huq et al., 1983, 1984; Tamplin et al., 1990; Thomas et al., 2006). Conversely, a negative association was noted between copepods and Vibrio in the 63–200-μm fraction, which may reflect differences in copepod life stages between the fractions. The majority of copepods in the 63–200-μm fraction represented earlier life stages (naupliar and copepodite), which displayed a bimodal distribution, peaking in May and October 2006, when water temperatures were not optimal for the growth of most Vibrio spp. (Thompson et al., 2003a). These earlier copepod life stages also differ physiologically in that earlier stages are continuously molting and shedding attached vibrios in the process (Tamplin et al., 1990).
In addition to copepods, within the 63–200-μm plankton fraction, phytoplankton (diatoms and cyanobacteria) were shown to be significant correlates of Vibrio abundance. In both cases, an increase in the relative abundance of diatoms or cyanobacteria corresponded with a significant decrease in co-measured Vibrio concentration. V. cholerae have been shown to attach to certain cyanobacteria and other algal species (for example, Tamplin et al., 1990); however, the total Vibrio community may respond differently to the presence of phytoplankton and the inverse relationship may reflect a release of nutrients that corresponds with the decay of a diatom or cyanobacterial bloom (Middelboe et al., 1995; Riemann et al., 2000). The decline of the bloom could then stimulate an increase in secondary production by heterotrophic bacteria, including Vibrio (Lancelot and Billen, 1984; Pomeroy and Wiebe, 2001). In particular, cyanobacterial-derived organic matter has been reported as an important growth factor for V. cholerae and V. vulnificus (Eiler et al., 2007). Alternately, the increase in the abundance of certain cyanobacteria, which are known to produce a range of antibacterial metabolites, could have contributed directly to a decline in the growth of total culturable Vibrio in that plankton fraction (Lam et al., 2008; Singh et al., 2008).
Within the >200-μm-size fraction, the only other plankton type, besides copepods, significantly associated with Vibrio levels were decapods, primarily crab larvae. Among the plankton models described for this study, the decapod model was unique in that changes in parameters other than relative plankton abundance, namely temperature, salinity and the concentration of Vibrio in the water fraction, were the primary drivers of Vibrio abundance (that is, parameter estimates were higher for these parameters than for the relative abundance of decapods). The association with decapods may have been weak for two reasons: (1) decapods comprised a relatively small proportion of this fraction and (2) the occurrence of decapods coincided with warm water temperatures, which were also optimal for the growth of Vibrio. In other words, the magnitude of change in the abundance of decapods was relatively small compared with the magnitude of change occurring in the total culturable Vibrio counts; however, this result does not preclude the idea that a rare member of the plankton community could not have a profound affect on the Vibrio population.
Our results confirm the importance of temperature, salinity and DO as independent drivers of both free-living and plankton-associated Vibrio concentrations. In addition, we have shown that plankton composition plays an important and independent role as a driver of the total culturable Vibrio community in natural estuarine systems. These findings highlight the potentially complex relationship between seasonal shifts in plankton composition and the net effect on total Vibrio levels and suggest that Vibrio concentrations should be considered within the context of bloom formation and decline as well as with single-point determinations of plankton composition.
References
Baffone W, Tarsi R, Pane L, Campana R, Repetto B, Mariottini GL et al. (2006). Detection of free-living and plankton-bound vibrios in coastal waters of the Adriatic Sea (Italy) and study of their pathogenicity-associated properties. Environ Microbiol 8: 1299–1305.
Colwell RR, Grimes DJ . (1984). Vibrio diseases of marine fish populations. Helgol Mar Res 37: 265–287.
Colwell RR, Huq A, Islam MS, Aziz KMA, Yunus M, Khan NH et al. (2003). Reduction of cholera in Bangladeshi villages by simple filtration. Proc Natl Acad Sci USA 100: 1051–1055.
Eiler A, Gonzalez-Rey C, Allen S, Bertilsson S . (2007). Growth response of Vibrio cholerae and other Vibrio spp. to cyanobacterial dissolved organic matter and temperature in brackish water. FEMS Microbiol Ecol 60: 411–418.
Fandino LB, Riemann L, Steward GF, Long RA, Azam F . (2001). Variations in bacterial community structure during a dinoflagellate bloom analyzed by DGGE and 16 S rDNA sequencing. Aquat Microb Ecol 23: 119–130.
Grossart HP, Levold F, Allgaier M, Simon M, Brinkhoff T . (2005). Marine diatom species harbour distinct bacterial communities. Environ Microbiol 7: 860–873.
Heidelberg JF, Heidelberg KB, Colwell RR . (2002a). Bacteria of the γ-subclass proteobacteria associated with zooplankton in Chesapeake Bay. Appl Environ Microbiol 68: 5498–5507.
Heidelberg JF, Heidelberg KB, Colwell RR . (2002b). Seasonality of Chesapeake Bay Bacterioplankton species. Appl Environ Microbiol 68: 5488–5497.
Huq A, Xu B, Chowdhury MA, Islam MS, Montilla R, Colwell RR . (1996). A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl Environ Microbiol 62: 2508–2512.
Huq A, Colwell RR . (1996). Environmental factors associated with emergence of disease with special reference to cholera. East Mediterr Health J 2: 37–45.
Huq A, Sack RB, Nizam A, Longini IM, Nair GB, Ali A et al. (2005). Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl Environ Microbiol 71: 4645–4654.
Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR . (1983). Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45: 275–283.
Huq A, West PA, Small EB, Huq MI, Colwell RR . (1984). Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol 48: 420–424.
Lam C, Stang A, Harder T . (2008). Planktonic bacteria and fungi are selectively eliminated by exposure to marine macroalgae in close proximity. FEMS Microbiol Ecol 63: 283–291.
Lancelot C, Billen G . (1984). Activity of heterotrophic bacteria and its coupling to primary production during the spring phytoplankton bloom in the Southern Bight of the North Sea. Limnol Oceanogr 29: 721–730.
Lipp EK, Rivera ING, Gil AI, Espeland EM, Choopun N, Louis VR et al. (2003). Direct detection of Vibrio cholerae and ctxA in Peruvian Coastal water and plankton by PCR. Appl Environ Microbiol 69: 3676–3680.
Lipp EK, Rodriguez-Palacios C, Rose JB . (2001). Occurrence and distribution of the human pathogen Vibrio vulnificus in a subtropical Gulf of Mexico estuary. Hydrobiologia 460: 165–173.
Long RA, Azam F . (2001). Antagonistic interactions among Marine Pelagic Bacteria. Appl Environ Microbiol 67: 4975–4983.
Long RA, Rowley DC, Zamora E, Liu J, Bartlett DH, Azam F . (2005). Antagonistic interactions among marine bacteria impede the proliferation of Vibrio cholerae. Appl Environ Microbiol 71: 8531–8536.
Louis VR, Russek-Cohen E, Choopun N, Rivera ING, Gangle B, Jiang SC et al. (2003). Predictability of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol 69: 2773–2785.
Massad G, Oliver JD . (1987). New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl Environ Microbiol 53: 2262–2264.
Middelboe M, Søndergaard M, Letarte Y, Borch NH . (1995). Attached and free-living bacteria: production and polymer hydrolysis during a diatom bloom. Microb Ecol 29: 231–248.
Morris Jr JG . (2003). Cholera and other types of vibriosis: a story of human Pandemics and Oysters on the half shell. Clin Infect Dis 37: 272–280.
Panicker G, Call DR, Krug MJ, Bej AK . (2004). Detection of pathogenic Vibrio spp. in shellfish by using multiplex PCR and DNA microarrays. Appl Environ Microbiol 70: 7436–7444.
Pfeffer C, Oliver JD . (2003). A comparison of thiosulphate-citrate-bile salts-sucrose(TCBS) agar and thiosulphate-chloride-iodide(TCI) agar for the isolation of Vibrio species from estuarine environments. Lett Appl Microbiol 36: 150–151.
Pfeffer CS, Hite MF, Oliver JD . (2003). Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl Environ Microbiol 69: 3526–3531.
Pinhassi J, Sala MM, Havskum H, Peters F, Guadayol O, Malits A et al. (2004). Changes in bacterioplankton composition under different phytoplankton regimens. Appl Environ Microbiol 70: 6753–6766.
Pomeroy LR, Wiebe WJ . (2001). Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Aquat Microb Ecol 23: 187–204.
Randa MA, Polz MF, Lim E . (2004). Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl Environ Microbiol 70: 5469–5476.
Riemann L, Steward GF, Azam F . (2000). Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl Environ Microbiol 66: 578.
Singh PK, Prasanna R, Jaiswal P . (2008). Cyanobacterial bioactive molecules-an overview of their toxic properties. Can J Microbiol 54: 701–717.
Sochard MR, Wilson DF, Austin B, Colwell RR . (1979). Bacteria associated with the surface and gut of marine copepods. Appl Environ Microbiol 37: 750–759.
Tamplin ML, Gauzens AL, Huq A, Sack DA, Colwell RR . (1990). Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol 56: 1977–1980.
Thomas KU, Joseph N, Raveendran O, Nair S . (2006). Salinity-induced survival strategy of Vibrio cholerae associated with copepods in Cochin backwaters. Mar Pollut Bull 52: 1425–1430.
Thompson FL, Iida T, Swings J . (2003a). Biodiversity of vibrios. Microbiol Mol Biol Rev 68: 403–431.
Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF . (2003b). Diversity and dynamics of a north Atlantic coastal Vibrio community. Appl Environ Microbiol 70: 4103–4110.
Verity PG, Alber M, Bricker SB . (2006). Development of hypoxia in well-mixed subtropical estuaries in the southeastern USA. Estuaries and coasts 29: 665–673.
Acknowledgements
This work was funded by the National Oceanic and Atmospheric Administration (NOAA) (Oceans and Human Health Initiative, Grant no. NA04OAR4600203). We thank the Georgia Department of Natural Resources (Coastal Research Division), especially our field coordinators, B Good, W Hughes and D Guadagnoli, for organizing monthly collection trips.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turner, J., Good, B., Cole, D. et al. Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J 3, 1082–1092 (2009). https://doi.org/10.1038/ismej.2009.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2009.50
Keywords
This article is cited by
-
Spatial patterns of phytoplankton communities in an International Seabed Authority licensed area (COMRA, Clarion-Clipperton Zone) in relation to upper ocean biogeochemistry
Acta Oceanologica Sinica (2022)
-
Hydrological regulation of Vibrio dynamics in a tropical monsoonal estuary: a classification and regression tree approach
Environmental Science and Pollution Research (2021)
-
Effects of carbon source addition on microbial community and water quality in recirculating aquaculture systems for Litopenaeus vannamei
Fisheries Science (2020)
-
Assessment of bacterial pathogens on edible macroalgae in coastal waters
Journal of Applied Phycology (2020)
-
Vertical variation in Vibrio community composition in Sansha Yongle Blue Hole and its ability to degrade macromolecules
Marine Life Science & Technology (2020)