Abstract
Both intra- and interspecific interactions between microbes are likely to play an important role in determining the severity of microbial infections. Here, we study the impact of interactions between coinfecting opportunistic pathogens Staphylococcus aureus and Pseudomonas aeruginosa on both phenotypic and genetic changes in a P. aeruginosa social trait, the production of iron-scavenging siderophores. Siderophores are facultatively upregulated in response to iron limitation and play a key role in determining the virulence of microbial infections. Siderophore production is metabolically expensive to individual producers but benefits the group as a whole because siderophores can be used by all cells in the vicinity with siderophore receptors. Hence, populations of siderophore producers can be invaded by nonproducing cheats. Previous work has shown that P. aeruginosa can lyse S. aureus, supplying a source of free iron. We therefore hypothesized that the presence of S. aureus might result in facultative downregulation of siderophore production, and in turn, reduced selection for siderophore cheats. We tested this hypothesis by evolving P. aeruginosa in the presence and absence of free iron and S. aureus, in a fully factorial design. Iron had the expected effect: siderophore production was downregulated and cheats evolved less readily, but the presence of S. aureus instead increased facultative siderophore production and selection for cheats. This is probably because the S. aureus had the net effect of competing for iron, rather than acting as an iron source. This study demonstrates that interspecific competition can have a marked effect on intraspecific social interactions.
Similar content being viewed by others
Introduction
Pathogen virulence is often mediated by cooperative traits (see West et al., 2006 and references therein). Thus, understanding the evolution and ecology of cooperation in pathogenic microbes not only provides a useful model system for testing theories of the evolution of cooperation (for example in the studies by Griffin et al., 2004 and Harrison and Buckling, 2005), but can also generate useful predictions about disease pathology (for example in Harrison et al., 2006). This may be of particular importance when considering long-term, chronic infections, such as those commonly experienced by patients with cystic fibrosis (CF) (Gilligan, 1991; Lyczak et al., 2002). The opportunistic bacterium Pseudomonas aeruginosa is a particularly important pathogen of CF patients, persisting in the airways for many years (Cystic Fibrosis Foundation, 2004).
The production of iron-scavenging siderophores by bacteria and fungi is a classic example of cooperation via production of a ‘public good’; while, metabolically expensive to produce, siderophores potentially benefit all cells in the vicinity with siderophore receptors (Ratledge and Dover, 2000; Griffin et al., 2004; Wandersman and Delepelaire, 2004). For pathogenic species such as P. aeruginosa, siderophores are necessary virulence factors (Meyer et al., 1996; Nyilasi et al., 2005; Harrison et al., 2006). This is because in aerobic conditions, iron exists in the insoluble Fe3+ form (Ratledge and Dover, 2000) and within animal hosts, it is usually complexed with high-affinity, iron-binding proteins (Payne, 1993).
Changes in siderophore production can be both phenotypic and genetic. Siderophore production is facultatively upregulated in response to iron starvation and decreases when populations are supplemented with iron (Ratledge and Dover, 2000). Genetic mutations can alter the maximum level of siderophore produced, or the shape of the siderophore production response to environmental iron concentration. While populations of siderophore producers will outperform populations of nonproducers, there is a strong advantage to social defection within cooperating populations. Siderophore nonproducers pay none of the costs of siderophore production but benefit from the siderophores produced by their neighbours, allowing them to increase in frequency. Such cells grow better in the presence of siderophore producers than they do in pure culture, and so may be termed social ‘cheats’ (Griffin et al., 2004). Cheating mutants rise to high frequencies under conditions of local competition and/or low relatedness, when kin selection for cooperation is diminished (Hamilton, 1964; Griffin et al., 2004).
Putting these two phenomena—environment-dependent gene regulation and natural selection on individuals—together leads to an interesting prediction. In a low-iron environment, where siderophore production is upregulated, the pool of available siderophore creates a selective advantage for cells that lose the ability to produce siderophore in response to low-iron signals, that is, cheats. After the initial physiological response to the environment, siderophore production should gradually decrease if the population is allowed to evolve over many generations, due to cheat evolution. The evolutionarily stable strategy level of cheating will be determined by population structure according to kin selection theory (West and Buckling 2003; Griffin et al., 2004). In an iron-enriched environment, siderophore production will also decrease, but this will most likely be due only to physiological downregulation of siderophore genes. There will be no selective advantage in losing the ability to produce siderophores if siderophores are not expressed.
Intraspecific competition between cooperators and cheats is not the only type of competition that may affect public goods production. Interspecific interactions may also mediate the cost/benefit ratio of siderophore production. In the CF airways, P. aeruginosa exists alongside numerous other pathogenic microbes. Its most notable coinfection partner is Staphylococcus aureus (Hoiby, 1974, 1982; Petersen et al., 1981; Santana et al., 2003; Anzaudo et al., 2005; Moore et al., 2005). P. aeruginosa is capable of lysing cells of S. aureus and utilizing the iron released to support its own growth in vitro (Mashburn et al., 2005; Palmer et al., 2005). It is not known how significant this extra iron is in the context of the CF lung, or how this behaviour is related to interspecific competition for environmental iron. We wished to determine whether P. aeruginosa responds to S. aureus as a competitor for iron or as an iron source. Further, we wished to determine whether any effect of S. aureus on P. aeruginosa siderophore production could be dependent on the levels of environmental free iron, as tissue damage due to chronic infection leads to elevated free iron levels in colonized airways (Britigan et al., 1993; Stites et al., 1998, 1999).
We found forty-eight populations of P. aeruginosa in broth microcosms. Populations were grown in the presence or absence of 106 colony-forming units (CFUs) of S. aureus and supplemented either with iron or with an iron chelator (human apotransferrin), in a fully factorial balanced design. This allowed us (a) to compare the effects of S. aureus and environmental iron and (b) to test for any interaction between the two treatments. Every day, 0.1% of each culture was transferred to a fresh microcosm and this serial passage was continued for 20 days (approximately 140 P. aeruginosa generations). Competition was therefore entirely local within this design, conferring an advantage to cheats. Thus, our experiment looked for any effect of interspecific interactions over and above that of intraspecific competition. Fresh ancestral S. aureus was added to microcosms each day; thus, this experiment did not address any effect of S. aureus evolution.
Materials and methods
Strains
The tetracycline-resistant P. aeruginosa strain PAO985 and the S. aureus clinical isolate FZ21 (a methicillin-resistant genotype) were used for this study. We confirmed that PAO985 could lyse FZ21 by dropping 1-μl spots of fresh overnight PAO985 cultures onto agar plates that had been thoroughly swabbed with fresh overnight cultures of FZ21. After incubation overnight at 37 °C, zones of inhibition were visible as clear rings in the S. aureus lawn surrounding P. aeruginosa colonies. We assayed FZ21 supernatants for siderophore production using the method described below, and found that this strain produces siderophores at a level undetectable by this assay, that is, at a level that is insignificant compared with the amount of siderophore produced by ancestral PAO985 (data not shown). Thus, our siderophore measures should reflect only siderophores produced by PAO985.
Growth conditions
All populations were grown in glass universal tubes containing 6 ml casamino acids medium (CAAs: 5 g casamino acids, 1.18 g K2HPO4.3H2O, 0.25 g MgSO4.7H2O, per litre) supplemented with sodium bicarbonate (necessary for iron chelator activity: Meyer et al., 1996) to a final concentration of 20 mM. Tubes were made iron limited by the addition of 70 μg ml−1 human apotransferrin (Sigma-Aldrich, UK), or supplemented with Fe(III)Cl3 to a final concentration of 5 μM. Supplementation with S. aureus was achieved by inoculating tubes with approximately 106 overnight culture cells of S. aureus.
The initial response of P. aeruginosa to the treatments was assayed by setting up 48 microcosms. Twenty-four were made iron limited and 24 supplemented with iron. Twelve tubes from each iron regime were supplemented with S. aureus, yielding a fully cross-factored design. c. 106 overnight culture cells of P. aeruginosa were then added to each tube and populations incubated for 24 h at 37 °C on an orbital shaker at 170 r.p.m. Per capita siderophore production and population density were measured as outlined below. The founding populations were also assayed for density and total siderophore production. The number of cell doublings in each microcosm was also calculated using the following formula:

where D1 and D2 represent the total CFU present at the beginning and the end of the growth period, respectively.
For the evolution experiment, 48 populations were set up in exactly the same manner as for the initial response experiment. Populations were incubated for 24 h (approximately seven P. aeruginosa generations) at 37 °C on an orbital shaker. Each population was then homogenized using a vortex mixer and 6 μl of culture was transferred to a new microcosm with fresh sodium bicarbonate, apotransferrin, iron and/or 106 fresh overnight cells of S. aureus as appropriate. Preliminary work had shown that the likelihood of transferring the remaining S. aureus into fresh tubes was minimal (the frequency of S. aureus cells after 24 h growth with P. aeruginosa as described above was ⩽4% in tubes sampled). This evolution was continued for 20 transfers. Every fifth day, the density and per capita siderophore production of each culture were measured as outlined below, and aliquots of the cultures were stored at −80 °C in 20% glycerol. The founding populations were also assayed for density and total siderophore production. Frozen population samples were later thawed and 10 μl of the mix added to 6 ml casamino acids containing 20 mM sodium bicarbonate and 70 μg ml−1 apotransferrin (common environment test). Cultures were incubated for 18 h at 37 °C on an orbital shaker, homogenized and the per capita production of siderophores was assayed as outlined below.
Our growth conditions create relatively low-relatedness populations undergoing a high level of local competition (Griffin et al., 2004). Thus, cheats could eventually evolve and rise to appreciable frequencies in all treatments, regardless of iron regime. Iron supplementation could simply cause a delay in this evolutionary response. However, over the relatively short time period of this experiment, we would expect to be able to see the effect of iron. In natural (environmental and clinical) populations, there will always be some degree of global competition (Saccheri and Hanski, 2006; Harrison, 2007) and this will allow kin selection for cooperation to operate when the environment sets a favourable cost/benefit ratio, that is, differences in cost/benefit ratio will be critical in determining the selective advantages of cooperation and cheating.
Assays
-
1)
Aliquots of diluted culture were plated on King's medium B agar to score total density. S. aureus colonies were never observed on plates that contained 30–300 colonies of P. aeruginosa.
-
2)
An aliquot of the whole-population mix was centrifuged to pellet the cells, and the supernatant (containing siderophores) was stored at −20 °C. The total siderophore content of these supernatants was later determined using the chrome azurol S (CAS) method described by Schwyn and Neilands (1987), with the modification that we diluted Schwyn and Neilands's CAS recipe 1:1 with double distilled H2O. The relative absorbance at 630 nm of a mixture of 50 μl supernatant and 100 μl CAS solution (all chemicals from Sigma-Aldrich, UK) decreases linearly as siderophore concentration rises. Thus, a measure of mean siderophore production per CFU in the ith microcosm is given by
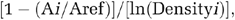
where Ai=absorbance of the ith sample, Aref=absorbance of a reference solution comprising 50 μl sterile growth medium plus 100 μl CAS and density=CFU in 50 μl of the population sample (all chemicals from Sigma). We have previously shown (Harrison and Buckling, 2005) that the mean per capita siderophore production as measured by the CAS assay is negatively correlated with the number of cheating clones as scored visually on iron-limited agar.
Statistical analyses
All data were analyzed using Minitab 14. All in situ siderophore data were arcsine square root transformed prior to regression analysis. Mean siderophore production over time was calculated using time points 5–20 and arcsine square root transformed. Density data were square root transformed prior to regression analysis. Data from time point zero were excluded from the calculation of mean density. For the analysis of initial responses, siderophore production data were squared prior to analysis. Nonsignificant interactions were removed from models where main effects were considered.
Results
Initial response to treatment environments
In this study, we wished to separate the physiological response of bacteria to an altered iron regime from any longer term, evolutionary response to selection. To determine the physiological response of P. aeruginosa to the treatment environments prior to any evolutionary change, we set up 48 microcosms containing either iron-limited or iron-supplemented growth medium, supplementing half with S. aureus. We inoculated these microcosms with ancestral P. aeruginosa and allowed the populations to grow for 24 h. Consistent with iron-dependent regulation of siderophore expression (Ratledge and Dover, 2000), iron-limited cultures produced significantly more siderophores per CFU than did iron-supplemented cultures (Figure 1a). (general linear model (GLM): F(1,43)=34.34, P<0.001). The effect of S. aureus was not significant (F(1,43)=1.09, P=0.303), but there was a significant interaction between iron and S. aureus (F(1,43)=5.21, P=0.027) such that siderophore production in iron-limited microcosms was higher when S. aureus was present (Figure 1a). This suggests that, in the short term, competition for iron occurs between the two species. As would be expected, populations in iron-supplemented conditions grew more rapidly, as measured by the number of cell doublings (F(1,44)=49.08, P<0.001). There was no significant effect of S. aureus on P. aeruginosa growth (F(1,44)=0.13, P=0.715).
(a) Initial, physiological modulation of siderophore production in response to treatment environments. Fe=iron, SA=S. aureus. Bars show mean±1 s.e. The dotted line shows siderophore production of the ancestral clone immediately prior to inoculation of experimental microcosms. This clone had been grown in casamino acids broth with neither iron nor iron chelator added. Mean per capita production of siderophores was lower under iron supplementation (P<0.001). When iron was limiting, mean siderophore production was increased in the presence of S. aureus (interaction P<0.05). (b) Mean levels of per capita siderophore production by evolving P. aeruginosa populations, as assayed in a common environment (iron-limited broth). Fe=iron, SA=S. aureus. Bars show mean±one s.e. Mean per capita production of siderophores was increased under iron supplementation (P<0.005). When iron was limiting, mean siderophore production was decreased in the presence of S. aureus (interaction P<0.05).
P. aeruginosa evolution experiment
We hypothesized that early upregulation of siderophore genes in iron-limited environments creates a selection pressure for social cheating and the appearance of siderophore-negative cheating mutants. When siderophore expression is downregulated in iron-rich environments, no such pressure exists, and cheating mutants are not expected to reach a significant frequency. However, if we attempt to look at siderophore production in situ in our evolving populations, we will not be able to separate cells that have facultatively downregulated siderophore production from true cheats. Net siderophore production as measured in situ may not differ between treatments, even though it is determined by different pressures in different environments.
Indeed, when mean in situ siderophore production per CFU over time is analyzed, neither iron nor S. aureus have any significant effect (Figure 2; GLM with block included as a factor: iron F(1,44)=2.72, P=0.106; S. aureus F(1,44)=0.42, P=0.522). We also calculated regression coefficients of in situ siderophore production over time for each population. Analysis of these slopes showed that they were more positive in the presence of iron but were not affected by the presence of S. aureus (GLM with block included as a factor: iron F(1,44)=34.78, P<0.005; S. aureus F(1,44)=0.00, P=0.992).
We therefore sought to obtain data that reflected solely the evolutionary changes in siderophore production, that is, the evolution of siderophore cheats. To do this, we grew up aliquots of stored population samples overnight in a common environment. This was identical to the iron limited, no S. aureus treatment. Iron limitation will force cells that had hitherto downregulated siderophore expression to restart production; only cheating mutants will be unable to respond to this environment. Assaying siderophore production in these samples should therefore give a reliable estimate of the extent of cheat evolution. (We have previously shown (Harrison and Buckling, 2005) that the chemical assay employed for measuring total siderophore production gives comparable results to visual scoring of producer and nonproducer colonies.)
Mean per capita siderophore production over time was strongly influenced by iron regime, being higher in iron-supplemented treatments (Figure 1b) (GLM with block included as a factor: F(1,42)=105.44, P<0.005). While presence of S. aureus was not significant as a main effect (F(1,42)=0.50, P=0.483), there was a significant interaction between iron and S. aureus, such that mean siderophore production was lower in the presence of S. aureus when iron was limiting (Figure 1b; F(1,42)=4.96, P<0.05). This is consistent with the observations from the initial response experiment. Taken together, these results suggest that when iron is already scarce, competition for iron from S. aureus initially causes P. aeruginosa cells to upregulate siderophore production even further, and this increases the selective advantage to cheating mutants. In other words, interspecific competition can have a measurable effect on siderophore cooperation even when combined with (presumably considerable) intraspecific competition. Presumably, competition from S. aureus is not a problem for P. aeruginosa in environments that are iron rich: the presence of the second species does not cause a significant reduction in the amount of iron available to P. aeruginosa, does not affect siderophore expression levels and so does not alter the selection pressure on cheating mutants.
Treatment also affected population growth over the course of the evolution experiment. Density showed a mean increase over time in all treatments (sign tests for median slope value >0 all had P-values<0.02). Mann–Whitney tests on regression coefficients showed that density increased significantly more rapidly in iron-supplemented treatments (W=306, P<0.005). There was no main effect of S. aureus (W=616, P=0.571) and there was no interaction between iron and S. aureus, that is, presence or absence of S. aureus did not affect slopes within iron regimes (iron limited: W=171, P=0.237; iron-supplemented: W=155, P=0.795). If mean density over time is analyzed, similar results are obtained: mean density was higher in the presence of iron (blocked GLM: F(1,44)=118.27, P<0.001) but there was no significant effect of S. aureus (F(1,44)=2.78, P=0.103). This is consistent with our explanations of the observed effect of S. aureus on siderophore production. In iron-rich environments, the presence of S. aureus does not alter the iron status of P. aeruginosa cells and so the latter do not respond to the presence of the second species. In iron-poor conditions, S. aureus competes with P. aeruginosa for what limited iron is available. P. aeruginosa rapidly responds by upregulating siderophore production. This increases the efficiency with which P. aeruginosa can scavenge iron and restore P. aeruginosa population growth to the same level observed in the absence of S. aureus.
Discussion
These results show that siderophore cheats evolve de novo much more readily under iron-limited, as opposed to iron-rich, conditions, that is, when the environment sets a low cost/benefit ratio to production, creating a common pool of highly beneficial siderophore and hence a selective advantage to cheating mutants. In iron-rich environments, siderophore production is physiologically downregulated due to a high cost/benefit ratio; hence, there is little siderophore for cheats to exploit. This is consistent with previous work describing short-term competition experiments between siderophore producers and a siderophore-deficient mutant across a range of iron-limitation conditions (Griffin et al., 2004).
These results also show that P. aeruginosa siderophore cheats are more common in the presence of S. aureus when no exogenous iron is supplied. This is not consistent with the hypothesis that iron released by S. aureus lysis acts in the same way as exogenous iron—if this was the case, we would expect the effect of S. aureus to be in the same direction as the effect of exogenous iron, that is, fewer cheats in the presence of S. aureus in the low-iron treatments. This result is, however, consistent with the results of the initial response experiment, where the presence of S. aureus led to siderophore upregulation. We therefore suggest that competition for iron between these two species, when iron is limiting, leads to initial upregulation of siderophore genes by P. aeruginosa, generating selection for cheating in exactly the same manner as iron limitation does. We cannot entirely rule out the possibility that other interactions between S. aureus and P. aeruginosa are responsible for siderophore upregulation (cross-species quorum sensing, for example: Keller and Surette, 2006), but competition seems to be the most parsimonious explanation. As S. aureus had no effect on siderophore production by P. aeruginosa when iron was supplied, a direct effect of this species on siderophore production seems unlikely. Measurements of iron availability in the test media, or of the amount of iron taken up by P. aeruginosa cells in the presence and absence of S. aureus could shed more light on the nature of this interaction.
We have demonstrated that interspecific interactions can affect an intraspecific social trait. Competition for a resource may have the same effect as environmental paucity for that resource. The two species we studied have been showed to interact in several way, both synergistically and antagonistically (Hoiby and Hertz, 1981; Burns et al., 1998; Ratjen et al., 2001; Lyczak et al., 2002; Mashburn et al., 2005; Palmer et al., 2005; Qazi et al., 2006). The net effect of these interactions, how this could differ between environments and how it might affect the outcome of mixed infections, has not been addressed, but is a topic of considerable interest (Harrison, 2007). It would be interesting to see if S. aureus ever can serve as a significant iron source for P. aeruginosa, and whether this can ever outweigh the effects of simple competition. The role of S. aureus evolution in long-term mixed populations is also a matter for consideration, as is any effect of environmental heterogeneity. Experiments designed to explore questions such as these could add significantly to our understanding of the complexity of community interactions and the virulence of mixed infections.
The results of these experiments suggest that environmental factors can significantly alter the cost/benefit ratio of production of a public good (siderophores). Alterations in this ratio cause an environment-dependent, physiological change in the levels of investment in siderophore production. This in turn creates differing selection pressures for social cheating in different environments. These observations strongly suggest that simply measuring the levels of public good production in a given environment will not give a reliable indication of the extent of social defection as public good levels will be the result of both physiological and selection-driven changes, that is, noting that a bacterial isolate does not produce siderophore when grown on an agar plate does not tell us whether that clone is a true cheating mutant, or whether it has facultatively downregulated its production of siderophore. Samples of bacteria from different environments should be grown up in a common environment (where the public good of interest is advantageous) to make the comparisons in their levels of cooperative behaviour meaningful.
This realization is particularly important when trying to understand selection pressures on siderophore production by pathogens such as P. aeruginosa. Most CF patients become chronically colonized with this species (Cystic Fibrosis Foundation, 2004), and while siderophores are likely to be necessary for initial colonization (Meyer et al., 1996; Harrison et al., 2006), siderophore-negative P. aeruginosa clones are commonly recovered from the airways of chronically infected CF patients (De Vos et al., 2001). Although mutations in siderophore genes have been recorded in P. aeruginosa isolated from the airways (Smith et al., 2006), the relative levels of cheating versus downregulation have not been elucidated. On the one hand, decreases in relatedness and increases in local competition in the lung might select for cheating genotypes (West and Buckling, 2003; Griffin et al., 2004). On the other, increased iron availability in inflamed airways (Britigan et al., 1993; Stites et al., 1998, 1999) could trigger siderophore downregulation. The presence of coinfecting species in the CF airways (see Harrison, 2007 for a review) could also affect the pressures on P. aeruginosa siderophore production, as a result of either increased competition for iron or increased the availability of iron. Determining why and how siderophore production is downregulated in clinical populations may suggest ways to target treatments more effectively.
References
Anzaudo MM, Busquets NP, Ronchi S, Mayoral C . (2005). Microorganismos patógenos aislados en muestras respiratorias de niños con fibrosis quística. Rev Argent Microbiol 37: 129–134.
Britigan BE, Hayek MB, Doebbeling BN, Fick Jr RB . (1993). Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun 61: 5049–5055.
Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L et al. (1998). Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 27: 158–163.
Cystic Fibrosis Foundation (2004). Patient Registry 2004 Annual Report. Cystic Fibrosis Foundation: Bethesda, MD.
De Vos D, De Chial M, Cochez C, Jansen S, Tummler B, Meyer JM et al. (2001). Study of pyoverdine type and production by Pseudomonas aeruginosa isolated from cystic fibrosis patients: prevalence of type II pyoverdine isolates and accumulation of pyoverdine-negative mutations. Arch Microbiol 175: 384–388.
Gilligan PH . (1991). Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev 4: 35–51.
Griffin AS, West SA, Buckling A . (2004). Cooperation and competition in pathogenic bacteria. Nature 430: 1024–1027.
Hamilton WD . (1964). The genetical evolution of social behaviour I & II. J Theor Biol 7: 1–52.
Harrison F . (2007). Microbial ecology of the cystic fibrosis lung. Microbiology 153: 917–923.
Harrison F, Browning LE, Vos M, Buckling A . (2006). Cooperation and virulence in acute Pseudomonas aeruginosa infections. BMC Biol 4: 21.
Harrison F, Buckling A . (2005). Hypermutability impedes cooperation in pathogenic bacteria. Curr Biol 15: 1968–1971.
Hoiby N . (1974). Epidemiological investigations of the respiratory tract bacteriology in patients with cystic fibrosis. Acta Pathol Microbiol Scand [B] Microbiol Immunol 82: 541–550.
Hoiby N . (1982). Microbiology of lung infection in CF patients. Acta Paediatrica Scandinavica Supplementum 301: 33–54.
Hoiby N, Hertz JB . (1981). Quantitative studies on immunologically specific and non-specific absorption of Pseudomonas aeruginosa antibodies in serum from cystic fibrosis patients. Acta Pathol Microbiol Scand [C] 89: 185–192.
Keller L, Surette MG . (2006). Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol 4: 249–258.
Lyczak JB, Cannon CL, Pier GB . (2002). Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15: 194–222.
Mashburn LM, Jett AM, Akins DR, Whiteley M . (2005). Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J Bacteriol 187: 554–566.
Meyer JM, Neely A, Stintzi A, Georges C, Holder IA . (1996). Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun 64: 518–523.
Moore JE, Shaw A, Millar BC, Downey DG, Murphy PG, Elborn JS . (2005). Microbial ecology of the cystic fibrosis lung: does microflora type influence microbial loading? Br J Biomed Sci 62: 175–178.
Nyilasi I, Papp T, Tako M, Nagy E, Vagvolgyi C . (2005). Iron gathering of opportunistic pathogenic fungi. A mini review. Acta Microbiol Immunol Hung 52: 185–197.
Palmer KL, Mashburn LM, Singh PK, Whiteley M . (2005). Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol 187: 5267–5277.
Payne SM . (1993). Iron acquisition in microbial pathogenesis. Trends Microbiol 1: 66–69.
Petersen NT, Hoiby N, Mordhorst CH, Lind K, Flensborg EW, Bruun B . (1981). Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma—possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand 70: 623–628.
Qazi S, Middleton B, Muharram SH, Cockayne A, Hill P, O’Shea P et al. (2006). N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect Immun 74: 910–919.
Ratjen F, Comes G, Paul K, Posselt HG, Wagner TO, Harms K . (2001). Effect of continuous antistaphylococcal therapy on the rate of P. aeruginosa acquisition in patients with cystic fibrosis. Pediatr Pulmonol 31: 13–16.
Ratledge C, Dover LG . (2000). Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54: 881–941.
Saccheri I, Hanski I . (2006). Natural selection and population dynamics. Trends Ecol Evol 21: 314–347.
Santana MA, Matos E, do Socorro Fontoura M, Franco R, Barreto D, Lemos AC . (2003). Prevalence of pathogens in cystic fibrosis patients in Bahia, Brazil. Braz J Infect Dis 7: 69–72.
Schwyn B, Neilands JB . (1987). Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160: 47–56.
Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA et al. (2006). Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 103: 8487–8492.
Stites SW, Plautz MW, Bailey K, O’Brien-Ladner AR, Wesselius LJ . (1999). Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am J Respir Crit Care Med 160: 796–801.
Stites SW, Walters B, O’Brien-Ladner AR, Bailey K, Wesselius LJ . (1998). Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest 114: 814–819.
Wandersman C, Delepelaire P . (2004). Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58: 611–647.
West SA, Buckling A . (2003). Cooperation, virulence and siderophore production in bacterial parasites. Proc R Soc Lond B Biol Sci 270: 37–44.
West SA, Griffin AS, Gardner A, Diggle SP . (2006). Social evolution theory for microorganisms. Nat Rev Microbiol 4: 597–607.
Acknowledgements
This work was supported by the Royal Society (AB) and by the University of Oxford (Newton-Abraham studentship awarded to FH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
FH, AB and RCM conceived the study. FH carried out experimental work, analyzed the results and drafted the manuscript; JP carried out experimental work; and AB and RCM contributed to manuscript preparation.
Rights and permissions
About this article
Cite this article
Harrison, F., Paul, J., Massey, R. et al. Interspecific competition and siderophore-mediated cooperation in Pseudomonas aeruginosa. ISME J 2, 49–55 (2008). https://doi.org/10.1038/ismej.2007.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2007.96
Keywords
This article is cited by
-
Community composition drives siderophore dynamics in multispecies bacterial communities
BMC Ecology and Evolution (2023)
-
Iron acquisition strategies in pseudomonads: mechanisms, ecology, and evolution
BioMetals (2023)
-
Copper selects for siderophore-mediated virulence in Pseudomonas aeruginosa
BMC Microbiology (2022)
-
Environmental structure impacts microbial composition and secondary metabolism
ISME Communications (2022)
-
Influence of the densities and nutritional components of bacterial colonies on the culture-enriched gut bacterial community structure
AMB Express (2021)