Abstract
The distribution of genetic and phenotypic variation in both hosts and parasites over their geographic ranges shapes coevolutionary dynamics. Specifically, concordant host and parasite distributions facilitate localized adaptation and further specialization of parasite genotypes on particular host genotypes. We here compare genetic population structure of the cultivated fungi of the fungus-growing ant Apterostigma dentigerum and of the cultivar-attacking fungus, Escovopsis, to determine whether these microbial associations have evolved or are likely to evolve genotype–genotype specialization. Analyses based on amplified fragment length polymorphism (AFLP) genotyping of host cultivars and pathogenic Escovopsis from 77 A. dentigerum colonies reveal that populations of hosts and pathogens are not similarly diverged and that host and pathogen genetic distances are uncorrelated, indicating that genetically similar parasites are not infecting genetically similar hosts. Microbial bioassays between pathogens and cultivars of different genotypes and from different populations show little pairwise specificity; most Escovopsis strains tested can successfully infect all cultivar strains with which they are paired. These molecular and experimental data suggest that Escovopsis genotypes are not tightly tracking cultivar genotypes within the A. dentigerum system. The diffuse nature of this host–pathogen association, in which pathogen genotypes are not interacting with a single host genotype but instead with many different hosts, will influence evolutionary and ecological disease dynamics of the fungus-growing ant–microbe symbiosis.
Similar content being viewed by others
Introduction
Parasites specialize on hosts at numerous ecological scales. At the broad level, most parasites attack only particular taxonomic host groups (for example, birds, annual plants, bacteria); at the finest level, some pathogen genotypes are specialized at attacking only a narrow range of host genotypes (Carius et al., 2001; Schulenburg and Ewbank, 2004). Specialization is a consequence of reciprocal adaptation by sympatric hosts and parasites. As predicted by the Red Queen hypothesis, this can lead to local parasite adaptation, the increased ability of parasites to infect and utilize local hosts (Parker, 1985; Lively, 1989; Gandon et al., 1996; Kaltz and Shykoff, 1998; Lively and Dybdahl, 2000; Dybdahl and Storfer, 2003).
Whether specialization arises is contingent on a variety of complex ecological and evolutionary factors. In particular, gene flow across host and parasite metapopulations is expected to play an important role in the evolution of localized adaptation within populations (Price, 1980; Kaltz and Shykoff, 1998; Lively, 1999; Thompson, 1999; Gandon and Michalakis, 2002; Thompson and Cunningham, 2002; Criscione et al., 2005). In general, host and parasite migration among populations can provide novel genetic variation that natural selection can subsequently act on, creating the potential for local adaptation, but it can also counteract adaptation by introducing locally maladapted alleles (Slatkin, 1985). More specifically, theory predicts that it is the relative gene flow of hosts and parasites that is important in defining local adaptive processes. First, when both hosts and their parasite have low gene flow, and particularly when their patterns of gene flow are similar, local parasite adaptation is likely to arise (Price, 1980; Kirkpatrick and Barton, 1997; Gandon, 1998). When parasites migrate more freely than their hosts (that is, parasite populations are less genetically structured than host populations), parasites are also expected to be locally adapted to hosts (Kaltz and Shykoff, 1998), but when hosts experience higher gene flow than parasites, parasites may be better suited to attack allopatric hosts over sympatric ones, thus appearing locally maladapted (Gandon et al., 1996).
Therefore, given that gene flow plays a critical role in coevolutionary dynamics, to understand the evolution of host–parasite interactions, it is important to have information on the relative population structure of the two players within a system. To date, only a handful of studies have characterized the population genetic structure of natural host and parasite metapopulations across their geographic range (Mulvey et al., 1991; Dybdahl and Lively, 1996; Davies et al., 1999; Delmotte et al., 1999; Martinez et al., 1999; Jobet et al., 2000; Sire et al., 2001; Jerome and Ford, 2002; Johannesen and Seitz, 2003), a very small fraction of which involve microbial pathogens (Delmotte et al., 1999). Jobet et al. (2000), for example, found similar genetic differentiation between populations of the urban cockroach (Blatella germanica) and its nematode parasite (Blatticola blattae). Many other studies, however, have found asymmetrical patterns of genetic structuring in hosts and parasites. Delmotte et al. (1999), for instance, found that populations of the fungal pathogen Microbotryum violaceum were much more strongly differentiated than were populations of its host plant, Silene latifolia, while Dybdahl and Lively (1996) found that populations of trematode parasites (Microphallus sp.) were much less differentiated than those of their host snails (Potamopyrgus antipodarum).
The fungus-growing ant–microbe symbiosis provides a unique opportunity to study microbial host–pathogen population structure, parasite specificity and disease dynamics. In this insect agricultural system, more than 200 ant species in the Tribe Attini are known to cultivate fungus as their primary food source. When new colonies are formed, founding queens take a piece of fungus from their mother's colony to start new colonies. Each colony cultivates a single fungal strain (Poulsen and Boomsma, 2005; Gerardo, personal observation; Scott et al., manuscript in preparation), either in the family Lepiotaceae or Pterulaceae (Basidiomycota, Agaricales) (Chapela et al., 1994; Munkacsi et al., 2004). These cultivars are attacked by Escovopsis, a genus of microfungi (Ascomycota, Hypocreales) thus far only found in association with fungus-growing ant colonies (Currie et al., 1999; Reynolds and Currie, 2004). Though only two species of Escovopsis, E. Weberi and E. aspergilliodes, are currently formally described (Seifert et al., 1995), morphological and genetic diversity of Escovopsis strains suggests that the genus is comprised of many species (Gerardo et al., 2006a). Analyses at the broad, interspecific level indicate that the diverse Escovopsis types, as defined by morphology and genetics, are specialized: particular Escovopsis clades only attack specific clades of fungal cultivars (Currie et al., 2003; Gerardo et al., 2004, 2006a). This specificity is not maintained by transmission along with the host cultivar, as infection patterns suggest that Escovopsis spp. are horizontally transmitted via a yet unknown mechanism (Currie et al., 1999; Currie, 2001; Gerardo et al., 2004).
The outcome of cultivar–Escovopsis encounters, as well as other host–parasite interactions, is ultimately predicated upon adaptations on part of the host to resist parasite establishment and adaptations on part of the parasite to overcome host defenses and establish infection (Combes, 2004). In many systems, adaptive mechanisms on the part of the host and parasite are difficult to elucidate. In the fungus-growing ant–microbe symbiosis, however, recent studies of the microbes associated with the ant genus Apterostigma suggest mechanisms by which host cultivars can prevent some infections and mechanisms by which pathogenic Escovopsis can efficiently infect some hosts. Experimental bioassays interacting Escovopsis with cultivars raised by a range ant species indicate that these pathogens are attracted to chemical signals produced by their host cultivars and by closely related fungi (Gerardo et al., 2006b). This attraction to cultivar signals may increase the efficiency by which Escovopsis establishes infection. Conversely, the same Escovopsis strains are not attracted to, but are inhibited by, distantly related cultivars raised by other fungus-growing ants. These phenotypes, Escovopsis host-seeking and cultivar resistance, may limit this parasite to successfully infecting only the cultivars raised by a subset of fungus-growing ant species (Gerardo et al., 2006b). It is unknown whether these adaptive mechanisms could function to maintain tight intraspecific specificity of Escovopsis strains on strains of its natural host.
To date, we know little about intraspecific host–pathogen population dynamics in the fungus-growing ant symbiosis, as the few fine-scale genetic analyses of fungus-growing ant-associated microbes have focused only on the host cultivars in a single geographical region (in and around the Panama Canal zone) (Bot et al., 2001; Green et al., 2002; Poulsen et al., 2005; Mikheyev et al., 2007), rather than on both host and parasites across populations. Here, we use amplified fragment length polymorphism (AFLP) genotyping to characterize the population genetic structure of cultivars and Escovopsis pathogens isolated in tandem from the colonies of Apterostigma dentigerum over a large geographic-scale spanning Central and South America. We use these population genetic data to compare patterns of host and parasite genetic structure. We also examine the distribution of host and parasite genotypes to look for signatures of fine-scale parasite specialization. These molecular analyses are coupled with experimental bioassays pairing genetically diverse parasite and cultivar strains in an attempt to identify signatures of local adaptation and strain–strain specificity. By assessing both patterns of host and parasite distribution over large geographic scales and specificity of host–parasites interactions at finer scales, we gain insight into the evolutionary and ecological dynamics of the Apterostigma ant–microbe symbiosis.
Materials and methods
Study system and collecting
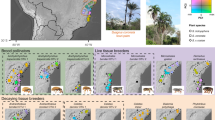
Colonies of A. dentigerum are common along stream banks and under logs throughout much of Central and South America (Gerardo, personal observation). Unlike subterranean ant colonies, A. dentigerum colonies can be easily detected and identified because of a conspicuous white veil of fungus that protects their internal fungal garden (Figure 1a; Villesen et al., 2004), facilitating collection of both garden material and ants from many colonies across populations.
Sampling of A. dentigerum colonies. (a) A. dentigerum colonies are easily located in the field because of the conspicuous, white fungal veil (see arrow) that protects their garden. After collection for this study, gardens were sampled to obtain pure isolates of the ants cultivated fungi (b) and the parasitic fungus Escovopsis (c). (d) Collecting sites and sample sizes. Cultivar and Escovopsis were collected from 77 colonies throughout Costa Rica, Panama, Ecuador and Argentina (the latter two are not shown). Stars mark the country capitals.
All A. dentigerum ants raise cultivars in the family Pterulaceae (Basidiomycota, Agaricales), which, based on DNA sequence analysis, appear to be genetically similar cultivar strains (Villesen et al., 2004). Within the context of a larger set of Apterostigma cultivar sequences, A. dentigerum cultivars are monophyletic at two genes (25S rDNA and COI) but not at ITS internal transcription spacer, a hypervariable marker. Although species boundaries have not been subscribed to fungal cultivars, the G2, G3 and G4 groups, in which Apterostigma cultivars are divided, are each considered well-supported monophyletic clades, and genetic differences within these clades, including the G2 group to which all A. dentigerum cultivars belong, are referred to as ‘strains’ or ‘types’ (Villesen et al., 2004) rather than as ‘species’.
These A. dentigerum cultivar strains are attacked frequently by three genetically and morphologically distinct parasite types: brown-spored, yellow-spored and white-spored Escovopsis (Gerardo et al., 2006a). All three Escovopsis types form monophyletic clades with little sequence divergence and are specific to attacking Apterostigma spp. cultivars (Gerardo et al., 2006a). Based on the morphological and sequence similarity within each clade, we refer to each Escovopsis type as a ‘species’.
Garden material from 472 A. dentigerum colonies was collected in 2001–2003 in Central and South America at 13 localities (Figure 1). At least 10 garden pieces (∼8 mm3) from each colony were grown on potato dextrose agar (PDA; Difco, Detroit, MI, USA) with bacteria-inhibiting antibiotics (50 mg/l each of penicillin and streptomycin), and pieces identified as either cultivar or as Escovopsis were subsequently subcultured to obtain axenic (pure) cultures before storage at −80°C.
From 77 of the 472 colonies collected, axenic cultures of both cultivar (Figure 1b) and brown-morphotype Escovopsis (Figure 1c) were isolated and stored successfully. Only samples from these colonies were included here, because use of host–parasite pairs isolated in tandem (that is, cultivar and Escovopsis from the same colony) assured that both hosts and pathogens were sampled similarly across space and time. Sample sizes for each population are listed in Figure 1. Escovopsis isolates of the yellow-spored and white-spored morphotypes were not included in the study, because Ef-1α sequences indicate that they are genetically distinct species from the brown-morphotype Escovopsis, and, furthermore, these pathogens are less common than the brown-morphotype, limiting sample size (Gerardo et al., 2006a).
AFLP genotyping
To examine the distribution of host and pathogen genotypes both across populations and relative to one another, we used AFLPs to profile cultivars and Escovopsis isolated from the same 77 colonies. Preceding amplification, DNA from a single cultivar and a single Escovopsis isolate from each colony was extracted according a cetyl trimethyl ammonium bromide protocol modified from Bender et al. (1983). Cultivar and parasite isolates from two randomly selected colonies were extracted twice and genotyped separately to detect the amount of noise (unreliable marker differences) generated during the amplification and scoring process. These duplicates were not included in graphical or statistical analyses.
For all samples, reactions followed the AFLP protocol for small plant genomes (www.appliedbiosystems.com; protocol 4303146) with the following modifications found to increase reliability of AFLP reactions: (1) before preselective amplification, 120 μl of TE0.1 was added to the restriction-ligation product instead of the recommended 189 μl, (2) the preselective product was not diluted for selective amplification, (3) for both preselective and selective amplifications, 25 thermocycler cycles were used instead of the recommended 20 cycles, (4) 3 μl of each selective product was mixed with 0.4 μl of GeneScan 500 Rox and 6.6 μl of Hi-Di formamide before generation of AFLP markers on an ABI Prism 3100 Genetic Analyzer. For selective amplifications, six combinations of AFLP-primer extensions were chosen because they generated high levels of polymorphic markers that could be scored reliably: AC/CAA, AC/CTG, AC/CTC, TG/CAG, TG/CTC and TC/CAG. Markers were scored blindly in Genotyper 2.5 by simultaneously comparing all fragments of a given length across all 77 Escovopsis isolates and, in a separate analysis, across all 77 cultivar isolates. Only markers that could be scored as unambiguously present/absent across all pathogen or host samples were used in analyses. At all stages, all cultivar and Escovopsis samples were run at the same time in 96-well plates and using the same reagents to minimize experimental artifacts.
Host and parasite population structures
For analysis of population structure, we performed parallel analyses of the cultivar (host) and Escovopsis (parasite) datasets and then compared results between the two. We conducted two analyses of molecular variance (AMOVA) in Arlequin (Schneider et al., 2000) to partition genetic variation both among host and among parasite isolates within and between localities. The AMOVA module in Arlequin generates Φ statistics, which is a molecular analog to Fisher's Fst (Excoffier, 2001). Population pairwise Φst values were also generated to determine the proportion of differences between hosts, and separately between parasites, associated with each locality. Levels of significance were determined through 100 000 random permutation replicates. Additional analogs of overall Fst (ΘB and GstB, an estimate of Nei's Gst (Nei, 1977) for diallelic loci) were calculated using Bayesian methods implemented in the program Hickory v1.0.4 (Holsinger et al., 2002). We ran all possible models available in Hickory, with default parameters recommended by the authors (Holsinger et al., 2002), but there were no substantial differences, so we report values obtained using the full model. For all population analyses, we excluded three localities at which only a single sample was collected (LSE, ARG and ELL), because no within-locality variation could be determined.
For hosts, and separately for parasites, we conducted Mantel tests in ZT (Bonnet and Van de Peer, 2002) to determine correspondence between each pairwise Φst (genetic distance) matrix and a pairwise geographical distance matrix. A significant, positive correlation would indicate effects of isolation by distance. All Mantel tests mentioned hereafter were also conducted using ZT and were performed with 10 000 permutations. Pairwise linear geographical distances between localities were calculated using the program Range (Luetgert). We also plotted the relationship between pairwise Φst and geographical distance for all pairs of host populations and all pairs of parasite populations.
To determine whether host and parasite populations exhibited a similar spatial pattern of divergence, we used Arlequin to construct two matrices: (1) the Nei's corrected average pairwise cultivar population differences; and (2) the Nei's corrected average pairwise Escovopsis population differences (Nei and Li, 1979). We then used a Mantel test to assess correspondence between the matrix of cultivar pairwise Φst values and parasite pairwise Φst values. A significant correlation would indicate that parasite populations show similar relative divergence to the host populations that they are attacking. We also visually compared cultivar population structure against parasite population structure using Structure v2.1 (Pritchard et al., 2000). Structure uses multilocus genotypic data to evaluate models of different numbers of clusters (K), with each individual probabilistically assigned to a reconstructed genetic cluster based on its multilocus genotype and the allele frequencies estimated for each cluster. Each genotype was treated as effectively haploid, where each locus contained one of two alleles determined by the presence or absence of a band. We applied a model of no-admixture and allele frequencies were assumed to be correlated within populations. Simulations were run from K=1 to K=9 with each K iterated five times, and we did not include any priors regarding sample location. To select the appropriate K, we calculated a ΔK statistic according to Evanno et al. (2005). Structure was also used to calculate FK values, interpreted as Fst analogs between each population and a single hypothetical common ancestral population (Pritchard et al., 2000; Falush et al., 2003). FK values were obtained by running the no-admixture model with K=10, utilizing locality as the population origin. All Structure simulations began with a burn-in of 100 000 and a runtime of 300 000.
Association of host and parasite genotypes
Correlation between host and parasite population pairwise differences would indicate that more genetically similar populations of hosts and parasites are associated, but this would not reveal parasite specialization at finer levels (that is, whether each parasite genotype within a population is attacking a narrow range of host genotypes within a population). To determine whether genetically similar parasites are attacking genetically similar hosts, we first visualized the relationships between the 77 cultivar isolates using mean character distances (that is, the sum of marker differences between two samples/total number of markers), generated in PAUP* (Swofford, 2002), to construct a nonmetric multidimensional scaling (NMDS) plot using NCSS (ver. 2000, Hintze, 2001). NMDS is an ordination technique that detects nonhierarchical structure by reducing the multidimensional relationship between entities to a smaller number of dimensions. A similar plot was created for the pathogen isolates. Mean character distances were used for these and all subsequent analyses, because though Nei–Li restriction distances (Nei and Li, 1979) are often selected for AFLP data analysis, many Escovopsis pair distances were undefined using this method. The Nei–Li and mean character distances for the cultivars were highly correlated (Mantel test, r=0.78, P<0.0001), however, and the results of no analysis were changed if the cultivar Nei–Li distances were used in place of the mean character differences. To visually verify the clustering produced through NMDS, we also used PAUP* to construct unweighted paired group means analysis (UPGMA) dendrograms.
In addition to visual inspection, we conducted two separate statistical analyses. First, we used a Mantel test to assess correlation between the host and parasite mean character difference matrices. Significance would indicate that more genetically similar parasite isolates (those with smaller mean character differences) attack more genetically similar host isolates, both between and within populations. Second, in both the NMDS plot and the UPGMA dendrogram, cultivar isolates fell into the same six visually distinct clusters. To verify the genetic distinctiveness of these clusters, we assigned each cultivar isolate to a cluster (clusters 1–6) and then used AMOVA to determine the proportion of the genetic variation among cultivar isolates. Pairwise comparisons among clusters were also conducted. Then, to determine whether the host cluster with which an Escovopsis isolate is associated could explain genetic variation among parasite isolates, we assigned each Escovopsis isolate to the cluster in which its host belonged and used these groups as a basis for AMOVA. A significant overall Φst would indicate the proportion of Escovopsis variation attributable to their association with genotypically distinct host clusters. We also conducted pairwise comparisons to determine which parasite groups, as defined based on host genotype cluster, were genetically differentiable. Significant pairwise differences would indicate cases in which genotypically differentiable parasite groups are attacking genotypically differentiable hosts.
Experimental test for specificity
We conducted microbial bioassays, following procedures in Gerardo et al. (2006b), to see if there is variation in the outcome of interactions between a set of genetically and geographically diverse A. dentigerum-associated Escovopsis strains and a set of equally diverse A. dentigerum-associated cultivar strains. For each bioassay, we placed a single isolate of cultivar near the edge of a 9 cm Petri dish with PDA+antibiotics. After 1 week, we inoculated the center of each plate with a single Escovopsis isolate. Each of nine cultivar isolates was interacted with each of the same nine Escovopsis isolates for a total of 81 bioassays. Experimental strains were chosen to maximize geographic and genetic diversity. Bioassays were monitored for up to 2 months. Interactions were scored for presence/absence of inhibition by the cultivar on Escovopsis growth and presence/absence of attraction by Escovopsis to the cultivar (Figure 5).
Microbial bioassays. Each cell represents the outcome of the interaction between one cultivar and one Escovopsis isolate. Gray indicates inhibition (i), a dot indicates attraction and subsequent infection (ii) and gray with a dot indicates attraction followed by inhibition (iii). The collection locality of each fungal strain is indicated in parentheses under its sample name.
To determine whether the variation that we observed in interactions between Escovopsis and cultivar isolates was associated with either genetic differences or geographic distances, we conducted a series of Mantel tests looking for the correlation between various distance matrices. Correlations with geographic distances would be consistent with local adaptation, while correlations with genetic distance would indicate specialization on particular genotypes. Four interaction distance matrices were constructed. The first matrix consisted of the inhibition distances between each pair of the nine cultivar strains, where each inhibition distance ranged from 0 to 1 and increased 0.11 for each case in which the two cultivar strains had a different inhibition result with the same Escovopsis strain (that is, one cultivar inhibited the Escovopsis strain while the other did not). A second matrix consisted of Escovopsis inhibition distances; each inhibition distance ranged from 0 to1 and increased 0.11 for each case in which the two Escovopsis strains had a different inhibition result with the same cultivar strain (that is, one Escovopsis strain was inhibited while the other was not). The third and fourth matrices were comprised of cultivar and Escovopsis attraction distances, which were determined similarly to the inhibition distances.
With the AFLP data, we constructed two genetic distance matrices using PAUP*: a mean character difference distance matrix for the nine experimental cultivar strains and a mean character difference distance matrix for the nine experimental Escovopsis strains. We also constructed two geographic distance matrices: a matrix of distances between collection sites of the cultivar strains and a matrix of distances between collection sites of the Escovopsis strains. We then used ZT to conduct Mantel tests to examine the correlation between matrices of (1) cultivar inhibition distances and cultivar genetic distances, (2) cultivar inhibition distances and cultivar geographic distances, (3) cultivar attraction distances and cultivar genetic distances, (4) cultivar attraction distances and cultivar geographic distances, (5) Escovopsis inhibition distances and Escovopsis genetic distances, (6) Escovopsis inhibition distances and Escovopsis geographic distances, (7) Escovopsis attraction distances and Escovopsis genetic distances and (8) Escovopsis attraction distances and Escovopsis geographic distances. To account for multiple tests, we applied a Bonferroni correction on all comparisons.
Results
AFLP diversity
For the host cultivars, a total of 804 AFLP markers were identified using the six primer systems; all are polymorphic and 208 (26%) are autapomorphic. For pathogenic Escovopsis, a total of 933 AFLP markers were identified; all are polymorphic and 334 (36%) are autapomorphic. Both cultivars and Escovopsis samples are diverse; mean character differences between cultivar isolates range from 0.02 to 0.29 (mean=0.16, s.d.=0.05), and mean character differences between Escovopsis isolates range from 0.04 to 0.22 (mean=0.14, s.d.=0.04). Of the four duplicated samples, the mean character difference between duplicates is low, ranging from 0.02 to 0.07 (mean=0.04, s.d.=0.02), and the difference between cultivar duplicates and between parasite duplicates is similar.
Host and parasite population structures
While both cultivar and Escovopsis populations exhibit little genetic structure along geographically determined boundaries, cultivar populations are relatively more geographically structured than those of their parasite. Among cultivar isolates, 11% of the variation is attributable to between-population differences (AMOVA overall Φst=0.11, Table 1a, ΘB=0.11, 95% CI 0.10–0.12), while 6%–7% of the variation among Escovopsis isolates is attributable to population differences (AMOVA overall Φst=0.07, Table 1a, ΘB=0.06, 95% CI 0.10–0.12). Estimates of GstB are very similar for cultivar (GstB=0.10, 95% CI 0.09–0.11) and Escovopsis (GstB=0.05, 95% CI 0.04–0.06).
Cultivar and Escovopsis population structures are shaped by geography but are not significantly correlated to one another. A Mantel test of the correspondence between cultivar pairwise Φst values and pairwise geographic distances confirms the effect of geographic isolation by distance (r=0.34, P=0.04). Escovopsis exhibits similar correlation between population genetic and geographic distances (r=0.38, P=0.03). This positive relationship between population genetic and geographic distances is represented in Figure 2. There is not, however, significant correspondence between cultivar population pairwise Φst values and Escovopsis population pairwise Φst values (r=0.22, P=0.139). This absence of corresponding population structure between host and parasite can be visualized by comparing the genetic clusters of each symbiont (Figures 3a and 3b). Consistent with the pattern of Escovopsis being slightly less structured than its host, as determined by the Fst analogs above, the genetic structure of Escovopsis conforms best to a model of K=3 genetic clusters, while the cultivar host is best explained by K=6. Though discrepancy in the values of K between the cultivar and Escovopsis does not necessarily translate to incongruent population genetic structure, as it is possible for finer-scale cultivar genetic structure to be nested within the three clusters of Escovopsis, we did not observe such a clustering pattern. For the cultivar, FK values (Figure 3c) are greatest in the Costa Rican population Hitoy Cerere Biological Station (HTC) (FK=0.12), followed by Fort Sherman in Panama (Fst, FK=0.10) while the most divergent Escovopsis population occurs in Barro Colorado Island (BCI, FK=0.10), the only region with a substantial FK for the pathogen. This implies that the most divergent host populations sampled do not correspond to the most divergent parasite population sampled, but it is important to note that these values, in general, are relatively low.
Isolation by distance. Plot of pairwise Φst/(1−Φst) against pairwise geographical distance between each of 10 populations of hosts (open circles) and parasites (filled circles). The relationship between genetic and spatial distances was assessed using Mantel tests and is significant for both cultivars (r=0.34, P=0.04) and Escovopsis (r=0.38, P=0.03).
Host and parasite population differentiation. (a) Genetic clusters for cultivars (K=6) and (b) for Escovopsis (K=3), determined using Bayesian methods in Structure and a ΔK statistic of Evanno et al. (2005). Each vertical line represents a single individual and each color corresponds to the genetic cluster to which an individual is probabilistically assigned. (c) FK values (an Fst analog between each population and a hypothetical common ancestor of all populations) for cultivars (white) and Escovopsis (black). Populations are arranged in order from northern Costa Rica to eastern Panama.
Association of host and parasite genotypes
Cultivars exhibit substantial genetic structure, as seen through the clustering of isolates in both the NMDS plot (Figure 4a) and UPGMA dendrogram (not shown) of all cultivar samples. Both clustering algorithms group cultivar isolates into six main clusters, and all isolates fall into the same cluster in both analyses. For the NMDS plot, dimension one captures 29% of the cultivar variation and dimension two captures an additional 22%. Upon a posteriori assignment of each of the cultivars to one of the six genotypic clusters, the resulting clusters account for 54% of the variation among isolates (Table 2a, AMOVA, overall Φst=0.54), substantially more than when the cultivars are assigned to populations (Table 1a, AMOVA, overall Φst=0.11). All six genotype clusters are significantly genetically distinct from one another (Table 2, pairwise differences). As can be seen in the Structure analysis (Figure 3b), these clusters do not correspond to geographically isolated populations.
Clustering of all host and parasite isolates. (a) Nonmetric multidimensional scaling (NMDS) solution for all cultivar isolates. Dashed lines demarcate six main host genotype clusters. Pairwise Φst comparisons indicate that all clusters are genetically differentiable (Table 2). (b) NMDS solution for all Escovopsis isolates. Isolates are coded by the cluster (1–6) of their associated host.
Escovopsis exhibits less genetic structure, with little clustering in the NMDS plot (Figure 4b) or UPGMA dendrogram (not shown). For the NMDS plot, dimension one captures 37% of the isolate variation, and dimension two captures an additional 15%. Lesser parasite clustering in Figure 4 as compared to host clustering suggests that there may be little concordance between host genetic similarity and parasite genetic similarity, and absence of tight parasite tracking of hosts genotypes is confirmed by a lack of significant correspondence between mean host differences and their respective mean parasite differences (r=0.04, P=0.065).
When Escovopsis isolates were assigned to groups based on the genotypic cluster with which their host cultivar was associated, this clustering explains a small, though significant portion of the variation among parasite isolates (Table 2b, overall Φst=0.025). Pairwise comparisons between groups of Escovopsis isolates that attack the different host genotype clusters indicate only one significant difference: parasites attacking cultivars in cluster one are only slightly, though significantly, genotypically distinct from parasites attacking cultivars in cluster three (Table 2, pairwise differences).
Experimental test for specificity
In the vast majority of bioassays, the Escovopsis isolate was attracted to the cultivar isolate, and the cultivar isolate was not able to inhibit the Escovopsis isolate (Figure 5). Of the eight Mantel tests looking for a correlation between an interaction distance matrix and either a genetic or geographic distance matrix, only one test had a P-value less than 0.1; there is a negative correlation between cultivar attraction distance and cultivar geographic distance (simple Mantel test: r=−0.3, P=0.04), likely driven by the fact that one cultivar isolate from Pipeline Road (PLR) inhibited an Escovopsis isolate not inhibited by the other two PLR cultivar strains. This weak correlation is not significant when a Bonferroni correction is applied.
Discussion
The distribution of parasite genotypes relative to their hosts, when analyzed over large geographic scales, gives insight into both local and global disease dynamics. Here, at a broad geographic scale, we see that host and parasite populations are not similarly differentiated. First, there is no correspondence between host and pathogen population pairwise Φst values, suggesting that hosts and pathogen populations are not diverging in tandem. Second, the most divergent host populations (indicated by FK) are not the same as the most divergent pathogen populations (Figure 3c). Furthermore, at a finer scale, we see no evidence of specialized pairings of host and parasite genotypes. Genetic analyses of neutral markers indicate that there is no correspondence between a matrix of host mean character differences and a matrix of pathogen mean character differences, and, whereas cluster analyses suggest several genetically distinct host clusters, there is no corresponding divergence in pathogen isolates (Figures 3 and 4). Supporting this lack of genotype–genotype specificity, experimental bioassays indicate that genetically distinct Escovopsis isolates are able to successfully infect a range of genetically and geographically diverse cultivar isolates (Figure 5), and there is no correspondence between genetic similarity of hosts and/or of parasites and infection success.
Many life-history traits of hosts and pathogens critically shape disease processes and likely lead to the patterns we see in the cultivar–Escovopsis system. Specifically, different modes of transmission increase the likelihood that parasite and host genotypes do not stay coupled across space and time (Huyse et al., 2005). In the fungus-growing ant system, previous epidemiological studies have suggested that while cultivars are vertically transmitted by the ants from a mother's colony to new colonies Escovopsis is independently horizontally transmitted (Currie et al., 1999). It has been hypothesized that Escovopsis could be vectored between colonies by microarthropods, or could be acquired by foraging ants either from the environment or during encounters with other ants (Currie, 2001; Gerardo et al., 2004). These mechanisms of disease spread likely have different barriers to gene flow than vertical transmission of the cultivar by the ants, and our results corroborate the existence of different barriers to gene flow for the host and parasite. Specifically, based on FK values (Figure 3c), the most divergent host population (HTC) in Costa Rica's Talamanca mountains, is not the most divergent parasite population (BCI), an island in the Panama Canal (Figure 1). While water would provide a barrier to Escovopsis migration to BCI by any of the above-mentioned horizontal transmission mechanisms, it would not prevent dispersal of the host cultivar by winged, migrating ants, which could easily fly the short distance from BCI to the mainland. On the other hand, cultivar-dispersing ants may have difficulty migrating to and from HTC because of their sensitivity to cooler temperatures in such higher-elevation regions. If different barriers to gene flow exist, then parasite genotypes would be less able to tightly track host genotypes.
Even without coupled dispersal, tight congruence of host–parasite population structures can be maintained by parasite specificity, such that parasites are only able to establish in a population if the appropriate host genotype is present. For example, the global population structure of Mycobaterium tuberculosis, which causes tuberculosis in humans, is defined by six lineages each associated with different human populations. In urban areas where different human populations have recently become sympatric, the M. tuberculosis strains historically associated with these human populations are present. This matching of human and pathogen distributions is shaped in part by tandem host–pathogen dispersal but also by coevolutionary adaptation. In a diverse urban host population, M. tuberculosis strains are much more likely to be infective in humans from populations with which they coevolved than in humans from other lineages (Gagneux et al., 2006). It is likely that specific host and parasite traits, namely host immune-related phenotypes and parasite virulence genes, maintain this specificity. We hypothesized that specific cultivar and Escovopsis traits would also maintain tight specificity of Escovopsis strains on cultivar strains. Host cultivars are known to be able to produce antibiotics that inhibit the growth of some Escovopsis spp., and Escovopsis spp. are known to efficiently recognize and establish infection on some hosts species (Gerardo et al., 2006b). Results here, however, suggest that these mechanisms are not facilitating genotype–genotype specificity or local adaptation at a population level, as a range of genetically and geographically diverse Escovopis strains were able to successfully infect a range of genetically and geographically diverse cultivar strains. Thus, these previously identified adaptive mechanisms (Gerardo et al., 2006b) may prevent a given Escovopsis from switching between host species but do not necessarily prevent Escovopsis from switching between different host strains.
The patterns of host and parasite association that we see here, driven by relatively independent host–parasite dispersal and a lack of genotype–genotype specificity, have implications for the coevolutionary dynamics of the cultivar–Escovopsis association. Specifically, focusing on the likelihood of Escovopsis to become adapted to local cultivar strains, theory suggests higher gene flow between parasite populations than between cultivar populations, particularly when coupled with substantial genetic structuring across host populations, will facilitate local adaptation of parasites on hosts (Gandon et al., 1996; Gandon and Michalakis, 2002). Although Escovopsis does exhibit slightly higher gene flow than cultivars, this discrepancy is likely not sufficient to support local adaptation, as the host cultivars exhibit little genetic population structure. As a comparison, in systems where population genetic analyses detected more extensive parasite than host migration and experimental work confirmed that parasites perform better on local than on novel host genotypes, the relative difference between parasite and host migration rates was much greater than is seen in this study. For example, Dybdahl and Lively (1996) found much higher levels of gene flow in trematode parasites relative to their snail hosts, and it has been experimentally demonstrated that these parasites are locally adapted to common host genotypes (Lively, 1989; Lively and Dybdahl, 2000). Similarly, Mutikainen and Koskela (2002) found higher parasite gene flow in parasitic plants than their perennial hosts, and these parasites had been previously reported to be locally adapted to their hosts (Koskela et al., 2000). In these cases, host populations were respectively 10 and 3 times more differentiated than their parasites, whereas here, with cultivar and Escovopsis, overall Φst values of host and parasite populations are less than twofold different. Moreover, results of Mantel tests between genetic and geographic distances indicate that the effects of isolation by distance in cultivars and Escovopsis are similar (Figure 3), and suggest that though there may be less parasite population differentiation at distances greater than 150 km (that is, when the natural log of geographic distance is greater than 5, Figure 3), at smaller distances, there is equal mixing between host populations as between parasite populations. Therefore, extensive local adaptation may be less likely in the cultivar–Escovopsis system than in other host–parasite associations where the difference between parasite and host genetic structure is greater.
Overall, it seems that local adaptation and strain-specific specialization is not likely to arise in the A. dentigerum cultivar–Escovopsis system. This is in contrast to the specificity that we see at broader scales, where Escovopsis species are constrained to attacking particular host species or groups of closely related species. This pattern of fine-scale lability underlying broad-scale specificity is consistent with other coevolutionary interactions. For example, a recent study of the host range of tropical plant pathogens found fungal pathogens were likely to be able to infect a range of closely related hosts, but were less likely to infect plants distantly related to their host. In other words, the likelihood of infection establishment decreased with increasing phylogenetic distance between the source host plant and the target plant species, with the steepest decline occurring in the most closely related plant pairs (Gilbert and Webb, 2007). Another example is the mutualistic association of sepiolid squid and their bioluminescent bacteria of the genus Vibrio. In this mutualism, phylogenetic congruence and coadaptation have been observed at a broad scale (Nishiguchi et al., 1998), but finer-scale genetic analyses show incongruent and low levels of genetic structuring across a geographic scale (Jones et al., 2006). The question remains as to how species specificity arises given the lability of associations at finer scales. Resolving this question will require coupling studies of specificity at multiple scales (among strains, among species, and so on) with studies of the genetics underlying host–parasite adaptations.
References
Bender W, Spierer P, Hogness DS . (1983). Chromosomal walking and jumping to isolate DNA from the Ace and Rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol 168: 17–33.
Bonnet E, Van de Peer Y . (2002). ZT: a software tool for simple and partial Mantel tests. J Stat Software 7: 1–12.
Bot ANM, Rehner SA, Boomsma JJ . (2001). Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutting ants. Evolution 55: 1980–1991.
Carius HJ, Little TJ, Ebert D . (2001). Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55: 1136–1145.
Chapela IH, Rehner SA, Schultz TR, Mueller UG . (1994). Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science 266: 1691–1694.
Combes C . (2004). Parasitism: The Ecology and Evolution of Intimate Interactions. University of Chicago Press: Chicago.
Criscione CD, Poulin R, Blouin MS . (2005). Molecular ecology of parasites: elucidating ecological and microevolutionary processes. Mol Ecol 14: 2247–2257.
Currie CR . (2001). Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 128: 99–106.
Currie CR, Mueller UG, Malloch D . (1999). The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci USA 96: 7998–8002.
Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Mueller UG et al. (2003). Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299: 386–388.
Davies CM, Webster JP, Kruger O, Munatsi A, Ndamba J, Woolhouse MEJ . (1999). Host–parasite population genetics: a cross-sectional comparison of Bulinus globosus and Schistosoma haematobium. Parasitology 119: 295–302.
Delmotte F, Bucheli E, Shykoff JA . (1999). Host and parasite population structure in a natural plant-pathogen system. Heredity 82: 300–308.
Dybdahl MF, Lively CM . (1996). The geography of coevolution: comparative population structures for a snail and its trematode parasite. Evolution 50: 2264–2275.
Dybdahl MF, Storfer A . (2003). Parasite local adaptation: Red Queen vs. Suicide King. Trends Ecol Evol 18: 523–530.
Evanno G, Regnaut S, Goudet J . (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611–2620.
Excoffier L . (2001). Analysis of population subdivision. In: Balding AR, Bishop M, and Cannings C (eds). Handbook of Statistical Genetics. John Wiley and Sons: New York, pp 271–307.
Falush D, Stephens M, Pritchard JK . (2003). Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164: 1567–1587.
Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S et al. (2006). Variable host–pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 103: 2869–2873.
Gandon S . (1998). Local adaptation and host–parasite interactions. Trends Ecol Evol 13: 214–216.
Gandon S, Michalakis Y . (2002). Local adaptation, evolutionary potential and host–parasite coevolution: interactions between migration, mutation, population size and generation time. J Evol Biol 15: 451–462.
Gandon S, Capowiez Y, Dubois Y, Michalakis Y, Olivieri I . (1996). Local adaptation and gene-for-gene coevolution in a metapopulation model. Proc R Soc Lond B Biol Sci 263: 1003–1009.
Gerardo NM, Mueller UG, Currie CR . (2006a). Complex host–pathogen coevolution in the Apterostigma fungus-growing ant-microbe symbiosis. BMC Evol Biol 6: 88.
Gerardo NM, Mueller UG, Price SL, Currie CR . (2004). Exploiting a mutualism: parasite specialization on cultivars within the fungus-growing ant symbiosis. Proc R Soc Lond B Biol Sci 271: 1791–1798.
Gerardo NM, Jacobs SR, Currie CR, Mueller UG . (2006b). Ancient host–pathogen associations maintained by specificity of chemotaxis and antibiosis. PLoS Biol 4: 1358–1363.
Gilbert GS, Webb CO . (2007). Phylogenetic signal in plant pathogen–host range. Proc Natl Acad Sci USA 104: 4979–4983.
Green AM, Mueller UG, Adams RMM . (2002). Extensive exchange of fungal cultivars between sympatric species of fungus-growing ants. Mol Ecol 11: 191–195.
Hintze J . (2001). NCSS. NCSS, Kaysville, UT.
Holsinger KE, Lewis PO, Dey DK . (2002). A Bayesian approach to inferring population structure from dominant markers. Mol Ecol 11: 1157–1164.
Huyse T, Poulin R, Theron A . (2005). Specification in parasites: a population genetics approach. Trends Parasitol 21: 469–475.
Jerome CA, Ford BA . (2002). Comparative population structure and genetic diversity of Arceuthobium americanum (Viscaceae) and its Pinus host species: insight into host–parasite evolution in parasitic angiosperms. Mol Ecol 11: 407–420.
Jobet E, Durand P, Langand J, Muller-Graf CDM, Hugot JP, Bougnoux ME et al. (2000). Comparative genetic diversity of parasites and their hosts: population structure of an urban cockroach and its haplo-diploid parasite (oxyuroid nematode). Mol Ecol 9: 481–486.
Johannesen J, Seitz A . (2003). Comparative population genetic structures of the fruit fly Urophora cardui and its primary parasitoid Eurytoma robusta. Entomologia Experimentalis Applicata 108: 149–157.
Jones BW, Lopez JE, Huttenburg J, Nishiguchi MK . (2006). Population structure between environmentally transmitted vibrios and bobtail squids using nested clade analysis. Mol Ecol 15: 4317–4329.
Kaltz O, Shykoff JA . (1998). Local adaptation in host–parasite systems. Heredity 81: 361–370.
Kirkpatrick M, Barton NH . (1997). Evolution of a species' range. Am Nat 150: 1–23.
Koskela T, Salonen V, Mutikainen P . (2000). Local adaptation of a holoparasitic plant, Cuscuta europaea: variation among populations. J Evol Biol 13: 749–755.
Lively CM . (1989). Adaptation by a parasitic trematode to local-populations of its snail host. Evolution 43: 1663–1671.
Lively CM . (1999). Migration, virulence, and the geographic mosaic of adaptations by parasites. Am Nat 153: S34–S47.
Lively CM, Dybdahl MF . (2000). Parasite adaptation to locally common host genotypes. Nature 405: 679–681.
Luetgert J . Range. United States Geological Service.
Martinez JG, Soler JJ, Soler M, Moller AP, Burke T . (1999). Comparative population structure and gene flow of a brood parasite, the great spotted cuckoo (Clamator glandarius), and its primary host, the magpie (Pica pica). Evolution 53: 269–278.
Mikheyev AS, Mueller UG, Boomsma JJ . (2007). Population genetic signatures of diffuse co-evolution between leaf-cutting ants and their cultivar fungi. Mol Ecol 16: 209–216.
Mulvey M, Aho JM, Lydeard C, Leberg PL, Smith MH . (1991). Comparative population genetic structure of a parasite (Fascioloides magna) and its definitive host. Evolution 45: 1628–1640.
Munkacsi AB, Pan JJ, Villesen P, Mueller UG, Blackwell M, McLaughlin DJ . (2004). Convergent coevolution in the domestication of coral mushrooms by fungus-growing ants. Proc R Soc Lond B Biol Sci 271: 1777–1782.
Mutikainen P, Koskela T . (2002). Population structure of a parasitic plant and its perennial host. Heredity 89: 318–324.
Nei M . (1977). F-statistics and analysis of gene diversity in subdivided populations. Ann Hum Genet 41: 225–233.
Nei M, Li WH . (1979). Mathematical-model for studying genetic-variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76: 5269–5273.
Nishiguchi MK, Ruby EG, McFall-Ngai MJ . (1998). Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-vibrio symbioses. Appl Environ Microbiol 64: 3209–3213.
Parker MA . (1985). Local-population differentiation for compatibility in an annual legume and its host-specific fungal pathogen. Evolution 39: 713–723.
Poulsen M, Boomsma JJ . (2005). Mutualistic fungi control crop diversity in fungus-growing ants. Science 307: 741–744.
Poulsen M, Cafaro M, Boomsma JJ, Currie CR . (2005). Specificity of the mutualistic association between actinomycete bacteria and two sympatric species of Acromyrmex leaf-cutting ants. Mol Ecol 14: 3597–3604.
Price PW . (1980). Evolutionary Biology of Parasites. Princeton University Press: Princeton.
Pritchard JK, Stephens M, Donnelly P . (2000). Inference of population structure using multilocus genotype data. Genetics 155: 945–959.
Reynolds HT, Currie CR . (2004). Pathogenicity of Escovopsis weberi: the parasite of the attine ant-microbe symbiosis directly consumes the ant-cultivated fungus. Mycologia 96: 955–959.
Schneider S, Roessli D, Excoffier L . (2000). ARLEQUIN: A Software for Population Genetics Data Analysis. Genetics and Biometry Laboratory, University of Geneva: Switzerland.
Schulenburg H, Ewbank JJ . (2004). Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol Biol 4: 49.
Seifert KA, Samson RA, Chapela IH . (1995). Escovopsis aspergilloides, a rediscovered hyphomycete from leaf-cutting ant nests. Mycologia 87: 407–413.
Sire C, Langand J, Barral V, Theron A . (2001). Parasite (Schistosoma mansoni) and host (Biomphalaria glabrata) genetic diversity: population structure in a fragmented landscape. Parasitology 122: 545–554.
Slatkin M . (1985). Gene flow in natural-populations. Ann Rev Ecol Syst 16: 393–430.
Swofford DL . (2002). PAUP*: Phylogenetic Analysis Using Parsimony (*: and Other Methods). Sinauer Associates: Sunderland, Massachusetts.
Thompson JN . (1999). Specific hypotheses on the geographic mosaic of coevolution. Am Nat 153: S1–S14.
Thompson JN, Cunningham BM . (2002). Geographic structure and dynamics of coevolutionary selection. Nature 417: 735–738.
Villesen P, Mueller UG, Schultz TR, Adams RMM, Bouck AC . (2004). Evolution of ant-cultivar specialization and cultivar switching in Apterostigma fungus-growing ants. Evolution 58: 2252–2265.
Acknowledgements
We thank S Xhao for her help with laboratory work, B Wee for help with analysis and interpretation, and C Currie and U Mueller for teaching us about fungus-growing ants. Research and export permits were granted by the Autoridad Nacional del Ambiente (Panama), Smithsonian Tropical Research Institute (Panama), Ministerio del Ambiente (Ecuador), Museo de Ciencias Naturales (Ecuador), Administración de Parques Nacionales (Argentina), Dirección de Conservación y Manejo (Argentina), Organization for Tropical Studies (Costa Rica) and Ministerio del Ambiente y Energia (Costa Rica). Critical logistical support was provided by M Leone (STRI), O Arosemena (STRI) and S Villamarin (Ecuador). Collections were made by C Currie, A Himler, L Laudermilk, A Mikehev, S Price, J Scott, A Smith and U Mueller. Helpful discussion and comments were provided by C Currie, U Mueller, M Singer, B Wee and three anonymous reviewers. This work was supported by NSF Doctoral Dissertation Improvement Grant DEB-0308757 and NSF IRCEB Grant DEB-0110073. N Gerardo was supported by fellowships from the University of Texas at Austin Graduate School and the UT Department of Integrative Biology, and E Caldera was supported by an NSF Graduate Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerardo, N., Caldera, E. Labile associations between fungus-growing ant cultivars and their garden pathogens. ISME J 1, 373–384 (2007). https://doi.org/10.1038/ismej.2007.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2007.57
Keywords
This article is cited by
-
Escovopsioides as a fungal antagonist of the fungus cultivated by leafcutter ants
BMC Microbiology (2018)
-
Symbiont-Mediated Host-Parasite Dynamics in a Fungus-Gardening Ant
Microbial Ecology (2018)