Abstract
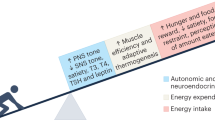
The relatively stable body weight during adulthood is attributed to a homeostatic regulatory mechanism residing in the brain which uses feedback from the body to control energy intake and expenditure. This mechanism guarantees that if perturbed up or down by design, body weight will return to pre-perturbation levels, defined as the defended level or set point. The fact that weight re-gain is common after dieting suggests that obese subjects defend a higher level of body weight. Thus, the set point for body weight is flexible and likely determined by the complex interaction of genetic, epigenetic and environmental factors. Unlike dieting, bariatric surgery does a much better job in producing sustained suppression of food intake and body weight, and an intensive search for the underlying mechanisms has started. Although one explanation for this lasting effect of particularly Roux-en-Y gastric bypass surgery (RYGB) is simple physical restriction due to the invasive surgery, a more exciting explanation is that the surgery physiologically reprograms the body weight defense mechanism. In this non-systematic review, we present behavioral evidence from our own and other studies that defended body weight is lowered after RYGB and sleeve gastrectomy. After these surgeries, rodents return to their preferred lower body weight if over- or underfed for a period of time, and the ability to drastically increase food intake during the anabolic phase strongly argues against the physical restriction hypothesis. However, the underlying mechanisms remain obscure. Although the mechanism involves central leptin and melanocortin signaling pathways, other peripheral signals such as gut hormones and their neural effector pathways likely contribute. Future research using both targeted and non-targeted ‘omics’ techniques in both humans and rodents as well as modern, genetically targeted, neuronal manipulation techniques in rodents will be necessary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kennedy GC . The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 1953; 140: 578–596.
Keesey RE, Powley TL . Hypothalamic regulation of body weight. Am Sci 1975; 63: 558–565.
Wirtshafter D, Davis JD . Set points, settling points, and the control of body weight. Physiol Behav 1977; 19: 75–78.
Harris RB . Role of set-point theory in regulation of body weight. FASEB J 1990; 4: 3310–3318.
Shin AC, Zheng H, Berthoud HR . An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav 2009; 97: 572–580.
Morgan PJ, Ross AW, Mercer JG, Barrett P . Photoperiodic programming of body weight through the neuroendocrine hypothalamus. J Endocrinol 2003; 177: 27–34.
Seeley RJ, Matson CA, Chavez M, Woods SC, Dallman MF, Schwartz MW . Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. Am J Physiol 1996; 271: R819–R823.
White CL, Purpera MN, Ballard K, Morrison CD . Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol Behav 2010; 100: 408–416.
Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR . Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 2010; 151: 1588–1597.
Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1273–R1282.
Mumphrey MB, Hao Z, Townsend RL, Patterson LM, Morrison CD, Munzberg H et al. Reversible hyperphagia and obesity in rats with gastric bypass by central MC3/4R blockade. Obesity (Silver Spring) 2014; 22: 1847–1853.
Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ . Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 2011; 31: 3904–3913.
Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD . Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 2001; 4: 605–611.
Mul JD, Begg DP, Alsters SI, van Haaften G, Duran KJ, D'Alessio DA et al. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab 2012; 303: E103–E110.
Grayson BE, Schneider KM, Woods SC, Seeley RJ . Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci Transl Med 2013; 5: 199ra112.
Hao Z, Zhao Z, Berthoud HR, Ye J . Development and verification of a mouse model for roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS One 2013; 8: e52922.
Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 2014; 306: R352–R362.
Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 2010; 138: 2426–2436, 2436 e2421-2423.
Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M et al. Very low calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell function in type 2 diabetic patients. Diabetes 2013; 62: 3027–3032.
Lips MA, de Groot GH, van Klinken JB, Aarts E, Berends FJ, Janssen IM et al. Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. Clin Endocrinol (Oxf) 2014; 80: 834–842.
Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010; 33: 1438–1442.
Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB et al. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond) 2012; 36: 348–355.
Schwartz MW . Brain pathways controlling food intake and body weight. Exp Biol Med (Maywood) 2001; 226: 978–981.
Keesey RE, Hirvonen MD . Body weight set-points: determination and adjustment. J Nutr 1997; 127: 1875S–1883S.
Levin BE, Keesey RE . Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol 1998; 274: R412–R419.
Hao Z, Munzberg H, Rezai-Zadeh K, Keenan M, Coulon D, Lu H et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J Obes (Lond) 2015; 39: 798–805.
Korner J, Conroy R, Febres G, McMahon DJ, Conwell I, Karmally W et al. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring) 2013; 21: 951–956.
Ravussin Y, Leibel RL, Ferrante AW Jr . A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab 2014; 20: 565–572.
Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008; 8: 169–174.
Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014; 124: 3913–3922.
Murphy M, Samms R, Warner A, Bolborea M, Barrett P, Fowler MJ et al. Increased responses to the actions of fibroblast growth factor 21 on energy balance and body weight in a seasonal model of adiposity. J Neuroendocrinol 2013; 25: 180–189.
Lelliott CJ, Ahnmark A, Admyre T, Ahlstedt I, Irving L, Keyes F et al. Monoclonal antibody targeting of fibroblast growth factor receptor 1c ameliorates obesity and glucose intolerance via central mechanisms. PLoS One 2014; 9: e112109.
Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KL et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab 2012; 97: E1023–E1031.
Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997; 88: 131–141.
Moore BS, Mirshahi UL, Yost EA, Stepanchick AN, Bedrin MD, Styer AM et al. Long-term weight-loss in gastric bypass patients carrying melanocortin 4 receptor variants. PLoS One 2014; 9: e93629.
Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C . Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg 2011; 21: 930–934.
Zechner JF, Mirshahi UL, Satapati S, Berglund ED, Rossi J, Scott MM et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology 2013; 144: 580–590 e587.
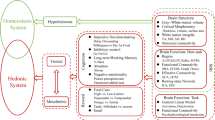
Berthoud HR . Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol 2011; 21: 888–896.
Shin AC, Zheng H, Pistell PJ, Berthoud HR . Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011; 35: 642–651.
Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S . Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 2014; 22: E13–E20.
Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L . Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc 1995; 95: 666–670.
Hajnal A, Kovacs P, Ahmed TA, Meirelles K, Lynch CJ, Cooney RN . Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 2010; 299: G967–G979.
Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014; 63: 891–902.
Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 2012; 209: 128–135.
Goldman RL, Canterberry M, Borckardt JJ, Madan A, Byrne TK, George MS et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring) 2013; 21: 2189–2196.
Atkinson RL, Brent EL . Appetite suppressant activity in plasma of rats after intestinal bypass surgery. Am J Physiol 1982; 243: R60–R64.
le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006; 243: 108–114.
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ . Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007; 3: 597–601.
Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716.
Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 2005; 90: 359–365.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007; 246: 780–785.
Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V . Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 2014; 3: 191–201.
Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 2013; 144: 50–52 e55.
Wilson-Perez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes 2013; 62: 2380–2385.
Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 2011; 60: 810–818.
Guan X, Shi X, Li X, Chang B, Wang Y, Li D et al. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab 2012; 303: E853–E864.
Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 2012; 22: 1084–1096.
Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B . Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008; 32: 1640–1646.
Culnan DM, Cooney RN, Stanley B, Lynch CJ . Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring) 2009; 17: 46–52.
Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis 2011; 29: 48–51.
Ahmad NN, Pfalzer A, Kaplan LM . Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013; 37: 1553–1559.
Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012; 153: 3613–3619.
Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S . Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab 2013; 98: E708–E712.
Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H . Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009; 58: 1400–1407.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 2009; 106: 2365–2370.
Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 2013; 98: 16–24.
Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 2013; 13: 514–522.
Osto M, Abegg K, Bueter M, le Roux CW, Cani PD, Lutz TA . Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav 2013; 119: 92–96.
Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM . Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013; 5: 178ra141.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470–1481.
Casselbrant A, Elias E, Fandriks L, Wallenius V . Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 2015; 11: 45–53.
Thaler JP, Choi SJ, Schwartz MW, Wisse BE . Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol 2010; 31: 79–84.
Berthoud HR . Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 2008; 20 (Suppl 1): 64–72.
Berthoud HR, Neuhuber WL . Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85: 1–17.
Shin AC, Zheng H, Berthoud HR . Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg 2012; 255: 294–301.
Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR . Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes Surg 2014; 24: 2145–2151.
Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg 2010; 20: 616–622.
Okafor PN, Lien C, Bairdain S, Simonson DC, Halperin F, Vernon AH et al. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obes Res Clin Pract 2015; 9: 274–280.
de Lartigue G, Ronveaux CC, Raybould HE . Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab 2014; 3: 595–607.
Acknowledgements
Partially funded by National Institutes of Health Grants DK047348 (HRB); DK 085495 and DK068036 (JY); DK092587 (HM); and DK081563 (CM). Publication of this article was sponsored by the Université Laval’s Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
H-RB has received lecture fees from Novo Nordisk, and grant support from the National Institutes of Health. CDM and HM have also received grant support from the National Institutes of Health. JY has received grant support from Suntory Foundation. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hao, Z., Mumphrey, M., Morrison, C. et al. Does gastric bypass surgery change body weight set point?. Int J Obes Supp 6 (Suppl 1), S37–S43 (2016). https://doi.org/10.1038/ijosup.2016.9
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijosup.2016.9
This article is cited by
-
Control of Eating Attributes and Weight Loss Outcomes over One Year After Sleeve Gastrectomy
Obesity Surgery (2024)
-
Epigenetic contribution to obesity
Mammalian Genome (2020)
-
DNA methylation screening after roux-en Y gastric bypass reveals the epigenetic signature stems from genes related to the surgery per se
BMC Medical Genomics (2019)
-
Serum IGF-binding protein 2 (IGFBP-2) concentrations change early after gastric bypass bariatric surgery revealing a possible marker of leptin sensitivity in obese subjects
Endocrine (2019)