Abstract
Oestrogen is essential for maintaining bone mass, and it has been demonstrated to induce osteoblast proliferation and bone formation. In this study, complementary DNA (cDNA) microarrays were used to identify and study the expression of novel genes that may be involved in MC3T3-E1 cells’ response to 17-β estradiol. MC3T3-E1 cells were inoculated in minimum essential media alpha (α-MEM) cell culture supplemented with 17-β estradiol at different concentrations and for different time periods. MC3T3-E1 cells treated with 10−8 mol⋅L−1 17-β estradiol for 5 days exhibited the highest proliferation and alkaline phosphatase (ALP) activity; thus, this group was chosen for microarray analysis. The harvested RNA was used for microarray hybridisation and subsequent real-time reverse transcription polymerase chain reaction (RT-PCR) to validate the expression levels for selected genes. The microarray results were analysed using both functional and pathway analysis. In this study, microarray analysis detected 5 403 differentially expressed genes, of which 1 996 genes were upregulated and 3 407 genes were downregulated, 1 553 different functional classifications were identified by gene ontology (GO) analysis and 53 different pathways were involved based on pathway analysis. Among the differentially expressed genes, a portion not previously reported to be associated with the osteoblast response to oestrogen was identified. These findings clearly demonstrate that the expression of genes related to osteoblast proliferation, cell differentiation, collagens and transforming growth factor beta (TGF-β)-related cytokines increases, while the expression of genes related to apoptosis and osteoclast differentiation decreases, following the exposure of MC3T3-E1 cells to α-MEM supplemented with 17-β estradiol. Microarray analysis with functional gene classification is critical for a complete understanding of complementary intracellular processes. This microarray analysis provides large-scale gene expression data that require further confirmatory studies.
Similar content being viewed by others
Introduction
Oestrogen, a major sex steroid hormone, has garnered considerable attention because of its importance in bone mass maintenance and the efficacy of hormone therapy in combating osteoporosis.1 Bone mass is maintained by balancing osteoblast-mediated bone formation and osteoclast-mediated bone resorption. Oestrogen deficiency leads to an increased rate of bone remodelling and tilts the balance between bone resorption and formation in favour of the former, resulting in bone loss and osteoporosis.2,3 Oestrogen deficiency is not only the dominant pathogenic risk factor for postmenopausal osteoporosis, but also the key factor that contributes to continuous bone loss in aging men.4
It has been well established by both in vivo and in vitro studies that oestrogen inhibits osteoclastic bone resorption.5 This phenomenon that has been variously attributed to the suppression of cytokine production in the bone microenvironment, which includes interleukin-1, interleukin-6, macrophage colony-stimulating factor, receptor activator of nuclear factor kappa-B ligand (RANKL) and tumour necrosis factor-α,6,7 and to the induction of apoptosis in osteoclasts.8 Bone remodelling is a complex cell–cell interaction; osteoblasts and osteoclasts are regulated by one another. The receptor activator of the nuclear factor kappa-B (RANK)–RANKL–osteoprotegrin (OPG) pathway was an important discovery that explained the mechanism by which the osteoblast lineage influences the activity and differentiation of osteoclasts.9 RANK is expressed on the surface of osteoclast precursors, RANKL is found on the surface of osteoblasts, and OPG, a ‘decoy receptor’ molecule, is released by osteoblasts. The activation and differentiation of osteoclast precursors into mature (active) osteoclasts requires the binding of RANKL to RANK. The RANK–RANKL interaction is inhibited by OPG, which competitively binds to RANKL.9 Previous studies have revealed that 17-β estradiol increases osteoprotegrin mRNA and protein levels in human osteoblastic cells in a dose- and time-dependent manner.10 Alternatively, a paracrine mechanism was found in which oestrogen affects osteoclast survival via upregulation of the Fas ligand in osteoblasts, leading to the apoptosis of osteoclast precursors.11
Oestrogen’s ability to prevent bone loss is also attributed to its ability to stimulate bone formation.2,12 Oestrogen receptors (ERs) on osteoblasts have been identified as potential target cells for oestrogen replacement therapy.13,14 Oestrogen helps preserve bone mass by increasing osteoblast proliferation and function.15 Postmenopausal women exposed to relatively high doses of oestrogen demonstrate sustained stimulation of osteoblast function.16 Furthermore, oestrogen prevents apoptosis of osteoblasts via the repression of apoptotic gene expression, thereby extending the life span of osteoblasts.17 Oestrogen deficiency also downregulates the transcription of the gene for insulin-like growth factor 1, reducing bone formation as a result.18 Various intermediary pathways have been found that participate in the oestrogen-mediated biological responses of osteoblasts, such as transforming growth factor (TGF)-β, the Wnt signalling pathways and apoptosis.
Although oestrogen’s various mechanisms of action on bone cells have been widely investigated, the studies are primarily focused on one or a small number of key factors; studies providing a complete picture from high-throughput data and signalling pathways via gene chips are rather limited. In this study, an in vitro experiment combined with a gene microarray technique was performed to identify novel genes that may be involved in MC3T3-E1 cells’ response to oestrogen. This large dataset can be examined for bioinformatics approaches that allow the examination of biological systems rather than alterations in individual genes, which will facilitate further understanding of the molecular mechanisms of oestrogen that affect osteoblasts.
Materials and methods
Cell culture
MC3T3-E1 osteoblast-like cells (ATCC, Manassas, VA, USA) were cultured in minimum essential media alpha (α-MEM) supplemented with 10% foetal calf serum (Invitrogen, New York, NY, USA) and 1% penicillin/streptomycin (Invitrogen, New York, NY, USA) at 37 °C in humidified air with 5% CO2. The cell culture medium was changed every 2–3 days.
Cell viability
Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s protocol. The cells were seeded in each well of a 96-well plate with 200 µL of culture media. The cells were incubated in the presence of 17-β estradiol at different concentrations (10−10, 10−9, 10−8 and 10−7 mol⋅L−1), and each concentration of 17-β estradiol was maintained for 1, 3, 5 and 7 days. A group that did not receive oestrogen treatment served as the control. At the end of the incubation period, the cells were incubated for 4 h with the MTT reagent at a final concentration of 0.5 g⋅L−1 followed by washing with phosphate-buffered saline (pH 7.4) and the addition of dimethyl sulfoxide. Complete dissolution was achieved after gentle shaking. The absorbances of the resulting solutions were recorded at 490 nm using a microplate spectrophotometer. Each condition was performed in quintuplicate.
Alkaline phosphatase assay
Alkaline phosphatase (ALP) activity was determined via enzymatic assay. Experiments were conducted 24 h after cells were seeded onto a six-well chamber. MC3T3-E1 osteoblast-like cells were treated with 17-β estradiol at different concentrations (10−10, 10−9, 10−8 and 10−7 mol⋅L−1), and each concentration of 17-β estradiol was maintained for 3, 5 and 7 days. A group that did not receive oestrogen treatment served as the control. After treatment, the cells were rinsed with phosphate-buffered saline and then lysed in buffer containing 10 mmol⋅L−1 Tris-HCl, pH 7.4, 0.2% Triton X-100 (Beijing Native Biological Technology, Beijing, China). The cell lysates were centrifuged, and the supernatants were used for the assays according to the manufacturer’s protocols. The optical density of p-nitrophenol at 520 nm was determined spectrophotometrically, and the results were normalized to the protein content of the sample, which was quantified using the bicinchoninic acid (BCA) protein assay kit (Keygenbio, Nanjing, China) in accordance with the manufacturer’s protocol.
Extraction of total RNA
The most appropriate concentration and incubation time of 17-β estradiol based on the results of the ALP assay and the MTT assay were applied to another group of MC3T3-E1 cells, which were cultured and processed as previously described. A Trizol Reagent kit (Invitrogen, New York, NY, USA) was used to extract total cellular RNA. The extracted RNA was stored at –70 °C. The purity of the isolated RNAs was determined by measuring the optical density (OD) value (A260/A280) using a spectrophotometer (Agilent, Shanghai, China).
Genome-wide oligonucleotide microarray analysis
Microarray analysis was performed using a Mus musculus 12×135k v2 array, which consists of 135 000 amino acid-modified 60-mer oligonucleotide probes representing 44 170 genes (Kangchen Bio Shanghai, China). After preparing 1 μg of DNase-treated total RNA, fluorescent dye (Cy3-dCTP)-labelled cDNA produced via RNA amplification and subsequent enzymatic reaction were then hybridized into an array using the NimbleGen Hybridisation System 4 (Roche NimbleGen, Madison, WI, USA). Finally, the arrays were scanned using a GenePix 4000B single-channel scanner (Molecular Devices, Sunnyvale, CA, USA), and the data were extracted from the obtained images using GenePix Pro v6.0 (Molecular Devices, Sunnyvale, CA, USA). GeneSpring v11.0 software (Agilent Technologies, Santa Clara, CA, USA) was used to analyse the genes that were differentially expressed between the experimental and control groups. The genes that were consistently altered in both arrays with differences in mean expression ratios that were greater than twofold on average were selected as differentially expressed genes. The microarray results were analysed via both gene ontology (GO) and pathway analysis using NimbleScan v2.5 Software (Roche NimbleGen, Madison, WI, USA). Each condition was performed in triplicate.
Quantitative real-time reverse transcription polymerase chain reaction
To confirm the microarray results, three representative genes were chosen at random for analysis with quantitative real-time reverse transcription chain reaction (RT-PCR). cDNA was prepared from 2 µg DNase-treated total RNA from either the test or the control sample using the First Strand SuperScript II Kit (Invitrogen, New York, NY, USA). Quantitative RT-PCRs were performed using the DNA Master SYBR Green I Kit and a LightCycler (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocols, and the results were analysed using LightCyler software version 3.5 (Roche Diagnostics, Mannheim, Germany). Glyceraldehyde-3-phosphate dehydrogenase gene (Gapdh) was used as a housekeeping gene. All data were reported as values normalized to Gapdh. A mathematical model reported by Pfaffl was employed to analyse the relative expression ratios of these genes.19 The relative expression ratio was determined using the formula E(CP1–CP2)gene/E(CP3–CP4)Gapdh, in which E represents the quantitative RT-PCR efficiency and CP is the crossing point. In the same samples, Gapdh was continuously expressed across the different physiologic time points. Primer sequences for each gene target are shown in Table 1. After reverse transcription, relative quantification of the selected genes was performed using a PCR Thermal Cycler (TaKaRa Biotechnology, Dalian, China). The reaction mixtures were incubated in the Applied Biosystems Rotor-Gene 3000 Real-time PCR System (Corbett Research, Sydney, Australia). The Peltier thermal cycler experimental protocol was performed as follows: denaturing program (95 °C for 5 min), amplification and quantification program with 40 cycles (95 °C, 10 s; 60 °C, 15 s; 72 °C, 20 s; 80 °C, 15 s with a single fluorescence detection), melting curve program (72 °C–99 °C, with a heating rate of 0.2 °C⋅s−1 and continuous fluorescence detection). Three parallel reactions were performed for each gene.
Additional microarray information
This microarray study followed the minimum criteria provided by the microarray experiment guidelines.20 Detailed protocols for RNA isolation, amplification, labelling and hybridisation can be provided by the authors upon request.
Statistical analysis
All experiments were repeated at least three times using different specimens, and the representative data of ALP was presented as the means±standard deviation. Significant differences among the MTT and ALP results were analysed using various analyses. Statistically significant differences in gene expression between each pair of experiments and the corresponding control samples were analysed using Lowess Normalisation and a paired t-test. Greater than 2.0-fold higher and less than 0.5-fold lower alterations in expression with a P<0.05 were considered significant.
Results
Cell viability based on the MTT assay
There were no significant differences in cell viability between the experimental groups and the control group on the first day (P>0.05). After 3 days, the cell viability of the groups treated with 10−9 and 10−8 mol⋅L−1 17-β estradiol was higher than that of the other two groups (10−7 mol⋅L−1 and 10−10 mol⋅L−1) (P<0.05); there was no significant difference between the 10−7 mol⋅L−1 group and the 10−10 mol⋅L−1 group (P>0.05); After 5 days, the group treated with 10−8 mol⋅L−1 17-β estradiol exhibited significantly higher cell viability than the other three experimental groups, and all four experimental groups performed significantly better than the control group. After 7 days, all groups exhibited trends that were identical to those that were detected after three days. When we compared the different time points of treatment with 10−8 mol⋅L−1 17-β estradiol, we found that the group that received 5 days of 17-β estradiol treatment exhibited the most significant difference in cell viability compared with the control group, which was significantly different compared with the other time points and concentrations (Figure 1).
Graph of MTT results for each group at different oestrogen concentrations and treatment periods (Mean±standard deviation, n =20). The MTT levels of the control group and the experimental groups at different oestrogen concentrations (10−10, 10−9, 10−8, 10−7 mol⋅L−1 17-β estradiol) and different durations. Error bars denote standard deviation. *P<0.05, compared with the control group; aP<0.05 compared with the other experimental groups of the same duration, but different oestrogen concentrations. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
ALP activity assay
As shown in Table 2, the group treated with 10−8 mol⋅L−1 17-β estradiol for 5 days displayed the highest level of ALP activity. There were no significant differences between the experimental groups and the control group on the third day (P>0.05). After 5 days of treatment, the ALP activity of the 10−8 mol⋅L−1 17-β estradiol group exhibited significantly higher ALP activity compared with the other three experimental groups. After 7 days of treatment, there were significant differences between the experimental groups and the control group (P<0.05), but there were no significant differences detected among each of the experimental groups (P>0.05).
RNA quality assurance via spectrophotometry
The OD A260/A280 ratio was approximately 2.0, which demonstrated that the RNA extracted from the cells was not degraded.
Gene microarray analysis
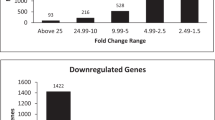
In this study, microarray analysis revealed 5 403 differentially expressed genes in the oestrogen-treated group compared with the control group. Of the differentially expressed genes, 1 996 were upregulated and 3 407 genes were downregulated. The differentially expressed genes related to bone metabolism in the oestrogen-treated group compared with those of the control group are presented in Table 3. Apoptosis and osteoclast differentiation-related genes were downregulated. Genes responsible for osteoblast proliferation and differentiation were upregulated. The genes that encode collagens (types II, IV, V, XI) and matrix metallopeptidases (MMPs) 14 and MMP2 were also upregulated.
Among the 1 553 different functional classifications related to GO analysis, a typical functional group is presented in Table 4, including GO terms, gene members, fold enrichment and the P value. Upregulated GO categories included cell differentiation, cell proliferation, intracellular signal transduction, cell communication and response to hormone stimulus. The downregulated categories included programmed cell death, apoptosis, and the Iκβ kinase/NF-κβ cascade.
Pathway analysis indicated that 53 pathways were upregulated and 67 pathways were downregulated. Upregulated pathways included the Wnt signalling pathway, the extracellular cell matrix (ECM) receptor interaction, the gap junction and the mitogen-activated protein kinase (MAPK) signalling pathways. There was some overlap between the downregulated and upregulated pathways, including the Wnt signalling pathway and the MAPK signalling pathway. Additionally, osteoclast differentiation and adherens junction pathways were downregulated. The corresponding pathways are presented in Table 5.
Confirmation of differential expression of selected genes via real-time RT-PCR analysis
The microarray results were validated using real-time PCR assays of selected genes. The findings confirmed the results obtained from the microarray analysis, despite slight disparity. Collectively, the results of the quantitative PCR demonstrated the reliability of the microarray analysis (Table 6).
Discussion
After menopause, reductions in the level of circulating oestrogen result in bone loss. Previous studies have confirmed that oral maxillofacial bone loss is closely associated with systemic osteoporosis. Oestrogen deficiency increases variability in the tissue mineral density of the alveolar bone surrounding the teeth during early remodelling 21 and decreases the osseointegration index in the implant/bone interface.22 In addition, 17-β estradiol has been shown to increase the osseointegration index at the implant/bone interface in osteoporotic rats.23
17-β estradiol is a type of artificially synthesized exogenous oestrogen with an activity and function similar to that of endogenous oestrogen, and it is often used to study the function of oestrogen. The MC3T3-E1 cell line, established from newborn mouse calvaria, is a useful model system for studying the mechanism of osteogenesis, as it possesses ERs (ERα and ERβ) and has been reported to retain the capacity to differentiate into osteoblasts.24 In this study, we applied different concentrations and durations of 17-β estradiol to MC3T3-E1 cells. Viability and proliferation were evaluated using the MTT assay, which may be a more sensitive method for assessing osteoblast proliferation because it measures cell viability via the determination of mitochondrial dehydrogenase activity.25 The proliferation of MC3T3-E1 cells reached its peak when subjected to 10−8 mol⋅L−1 17-β estradiol for 5 days. As the treatment period was extended further, the proliferation activity of the MC3T3-E1 cells tended to decline. These results are consistent with the experimental evidence reported previously.26,27 The differentiation of osteoblasts was assessed via ALP activity, an early marker of osteoblast differentiation.28 After 3 days of treatment, the ALP activity of MC3T3-E1 cells was low because the cells were still in the proliferation stage. The ALP activity of MC3T3-E1 cells reached its peak when the cells were subjected to 10−8 mol⋅L−1 17-β estradiol for 5 days; as the treatment period was extended, the ALP activity of MC3T3-E1 cells tended to decline. The MTT and ALP results indicate that the response of the MC3T3-E1 cells to 17-β estradiol varies according to the concentration and duration of oestrogen and the cell stage. Thus, the 10−8 mol⋅L−1 17-β estradiol group was chosen for the microarray analysis.
Genome-wide analysis via microarrays is a useful and complementary approach to genetic studies for the identification of genes and corresponding pathways that contribute to a given phenotype. An analysis of the genes with differential expression in MC3T3-E1 cells in response to 17-β estradiol could yield information about potential signal transduction pathways and candidate genes. Biological process analyses enable the further understanding of the differentially expressed genes. In this study, a total of 40 genes were involved in hormonal stimulus responses, and 174 genes were involved in intracellular signal transduction. Additionally, a number of routine biological processes exhibited elevated expression, including those responsible for cellular proliferation (Fgfr3, Calca, Apoe, Cav1, Eng, Vash1 and Cdh13) and cell differentiation (Lamb2, Mll2, Rps6ka2, Cav1 and Hey2). Fibroblast growth factor receptor 3 (FGFR3) is an important regulator of bone development and remodelling. Studies have indicated that FGFR3−/− mutations in mice result in decreased cortical bone thickness and osteomalacia.29 Therefore, these processes may imply an underlying molecular mechanism by which 17-β estradiol promotes the proliferation and differentiation of MC3T3-E1 cells.
Two hundred and six genes involved in apoptosis (Bcl2l11, Atp7a, Jun, Mapk9, Serpinb9, Tep1, etc.) were downregulated based on the biological processes analysis. Bradford et al.30 induced apoptosis of G-292 human osteoblasts and this activity was inhibited by pre-treatment with estradiol. They concluded that oestrogen combats osteoporosis by preventing osteoblast apoptosis and that a key event in this process is the repression of apoptotic gene expression. The downregulation of apoptosis-related genes may provide another functional effect of 17-β estradiol on osteoblasts.
It was reported that the beneficial effects of oestrogen on osteogenesis are caused in part by oestrogen’s ability to suppress osteoclastogenic cytokine production by osteoblasts.6,7 Thirty genes (Stat1, Ifnar, Ifngr, Chuk, Csf1r, Mapk9, Ncf1) related to osteoclast differentiation were also found to be downregulated in this experiment. Signal transducer and activator of transcription 1 (STAT1) is a critical regulator of both osteoclastogenesis and osteoblast differentiation in skeletal fracture healing. Studies reveal that STAT1 negatively regulates osteoblast differentiation by inhibiting the nuclear translocation of RUNX2 and by suppressing osterix transcript levels and promoter activity.31,32 These results indicate that STAT1 interferes with osteoblast-mediated bone formation. Interferons (IFNs) are important factors in the immune system. Studies of bone destruction associated with rheumatoid arthritis have highlighted the importance of the interaction between the immune and skeletal systems. Previous studies have indicated that IFN-β modifies human osteoblast function by inhibiting ECM synthesis, eventually resulting in delayed bone formation and mineralisation in the early phase of osteoblast differentiation.33 Oestrogen represses osteoclast activity via the downregulation of genes related to osteoclast differentiation, which may be another mechanism by which oestrogen promotes osteoblast proliferation.
The RANK–RANKL–OPG system is an essential signalling pathway involved in bone cell–cell communication, and it plays important roles in bone remodelling. OPG, a key modulator of the function of the RANK ligand, plays a significant role in the physiological regulation of and pathological alterations in bone metabolism.34 It has been demonstrated that OPG increases bone mass when the recombinant protein is administered to normal rats,35 and the deletion of the mouse Tnfrsf11b results in severe osteoporosis resulting from increased osteoclast activity.36 In this study, the expression of Tnfrsf11b was not significantly altered. Previous studies have indicated that the protein and mRNA expression of osteoprotegrin were significantly increased after 24 h of estradiol treatment, while smaller increases were observed with estradiol compared with untreated primary human osteoblast cells at extended time points.37 The lack of a significant difference in the expression level of Tnfrsf11b may be attributed to the cell system, or it may be caused by the extended duration of oestrogen treatment in our study.
The Wnt signalling pathway is one of the principal pathways participating in osteoblast differentiation and bone formation. Studies have revealed that Wnt pathway activation enhances osteoblast and osteocyte survival in vitro,38,39,40 and the Wnt pathway was active in bone regeneration sites.41 Oestrogen has also been implicated in the upregulation of Wnt protein and Frizzled receptor expression,42 and it induces the differentiation of uncommitted osteoblast progenitors by stimulating both Wnt and bone morphogenetic protein (BMP) signalling in a kinase-dependent manner in vitro and in vivo.43 In this study, microarray analysis identified the differentially expressed genes involved in Wnt; the expression of synthase kinase 3 beta (Gsk3β) reduced by 10 times, and the expression of van Gogh-like 2 (Vangl2) increased more than 10-fold. These results were confirmed via RT-PCR. GSK3β is a negative regulator of canonical Wnt signalling that phosphorylates the serine and threonine residues of β-catenin, leading to the degradation of β-catenin via a proteasome-dependent mechanism.44 Wnt signalling disrupts the GSK3β–Axin–APC–β-catenin complex via poorly understood mechanisms that may involve the recruitment of GBP/frequently rearranged in advanced T-cell lymphoma (Frat). GBP/Frat displaces Axin from GSK3β, leading to β-catenin release and stabilisation. Gaur et al.45 found that the canonical Wnt pathway promotes bone formation via the β-catenin/T-cell factor (TCF)-mediated activation of the master osteogenic transcription factor Runx2, which drives mesenchymal cells to an osteogenic lineage. In addition to the canonical Wnt signalling pathway, Vangl2, a non-canonical Wnt signalling gene, was also affected by the oestrogen treatment of MC3T3-E1 cells. Alysia found that a Vangl2 mutation in the loop-tail mouse uterine epithelium displayed altered cell polarity46 concomitant with changes in cytoskeletal actin and scribble localisation.
In addition to the Wnt signalling pathway, the MAPK pathway plays a key role in extracellular signal transduction to initiate cellular responses. MAPK pathways can relay, amplify and integrate signals from a diverse range of stimuli and elicit appropriate physiological responses, including cellular proliferation, differentiation, development, inflammatory responses and apoptosis. Studies have demonstrated that the MAPK signalling pathways activate and phosphorylate the osteoblast-specific transcription factor Cbfa1 in MC3T3-E1 cells and play an important role in the regulation of osteoblast-specific gene expression.47 Other studies suggest an important role for the extracellular signal-regulated kinase (ERK)–MAPK pathway in the regulation of osteoblast differentiation and foetal bone development.48 Alternatively, 17-β estradiol was found to induce osteoblast proliferation via the G protein-coupled receptor GPR-30, which is partly mediated by MAPK activation.
Connexin 43 (Cx43), which is expressed in virtually all types of bone cells, is the major component of gap junctions and plays important roles in signal transduction. Previous studies have revealed that the dynamic balance of bone mass was broken by reduced Cx43 expression rates in the osteoblasts and increased Cx43 expression rates in the osteoclasts of ovariectomized rats.49 Reportedly, oestrogen upregulates Cx43 expression in the human myometrium.50 Moreover, Cx43 expression was shown to be downregulated by oestrogen deficiency in myocardial cells in ovariectomized rats.51 Recent studies indicate that oestrogen not only increases Cx43 expression and the function of gap junction intercellular communications, but also enhances the mechanosensitivity of MLO-Y4 cells to mechanical loads via ERs.52 In this study, the expression level of Gja1 increased, which is consistent with previous studies. These findings all point to a possible link between oestrogen, Cx43 and Cx43-based gap junction intercellular communication. An elucidation of the interaction between oestrogen and Cx43 will deepen our understanding of the effects of oestrogen on bone metabolism.
BMPs are the most potent regulators of osteoblast differentiation among the local factors. Osteogenic protein 1, also referred to as BMP-7, belongs to the TGF-β superfamily. BMP-7-deficient mice exhibit polydactyly and occasional abnormalities of the ribs.53 Studies indicate that BMP-7 induces the expression of Cbfa1 mRNA in C3H10T1/2 cells.54 BMP-7 stimulates osteoblast differentiation by increasing collagen and osteocalcin synthesis, ALP activity and parathyroid hormone (PTH) responsiveness in ROS17/2.8 cells and MC3T3-E1 cells.55,56 BMP-7 also increases the formation of bone nodules by rat calvarial osteoblasts.57,58 Rickard et al. investigated the effects of oestrogen on BMP production using two oestrogen-responsive human immortalized osteoblast cell lines, hFOB/ER3 and hFOB/ER9.4 Interestingly, oestrogen (17-β estradiol: 10−10–10−7 mol⋅L−1) increased the expression level of BMP-6 mRNA and the production of BMP-6 protein, while BMP-7 mRNA levels were unchanged. In our study, Bmp7 was upregulated more than sevenfold in MC3T3-E1 cells treated with 10−8 mol⋅L−1 17-β estradiol for 5 days. The different cell types and culture conditions may account for the differences from the previous studies.
Aside from the role of osteoblasts in bone formation, they also express a number of matrix-degrading MMPs, including MMP-2, MMP-9, MMP-13 and MMP-14.59,60,61,62 Studies of Mmp null animals have defined important roles for osteoblast-derived MMPs. For example, Mmp-2 null mice have been shown to exhibit impaired skeletogenesis during development. These unexpected findings can in part be explained by the necessity of MMP-2 for osteoblast differentiation.63 However, the precise mechanism through which osteoblast-derived MMP-2 contributes to osteoblast function is unknown. MMP-14 is an important mediator of MMP-2 activation and could promote the secretion of MMP2; both of these are involved in osteoblastic bone formation and/or inhibit osteoclastic bone resorption.64 In this study, the expression of the Mmp-2 and Mmp-14 genes were increased in the oestrogen groups compared with the control group. This implies that 17-β estradiol likely promotes the proliferation and differentiation of MC3T3-E1 cells mediated by MMP-2 and MMP-14.
Conclusions
In conclusion, MC3T3-E1 cells responded to 17-β estradiol depending on the concentration and duration of treatment. Based on gene-chip analysis, a total of 5 403 genes were determined to be involved in the biological activities of osteoblasts either directly or indirectly under the influence of 17-β estradiol. These findings clearly demonstrate that the expression of genes related to osteoblast proliferation, cell differentiation, collagens and TGF-β-related cytokines increases, while the expression of genes related to apoptosis and osteoclast differentiation decreases following exposure of MC3T3-E1 cells to minimum essential media alpha supplemented with 17-β estradiol. Alternatively, we identified several novel genes involved in the response of MC3T3-E1 cells to 17-β estradiol. Previous studies demonstrated that many of these genes were involved in osteogenic pathways in osteoblasts or were implicated in the response to 17-β estradiol in other cell types.
Microarray analysis with functional gene classification plays an important role in providing a complete understanding of complementary intracellular processes, and the related genes or corresponding proteins may be explored in detail to identify more effective methods for treating osteoporosis. Furthermore, the obtained biological data could be critical for predicting the outcomes of strategies aimed at promoting bone regeneration, such as bone tissue engineering.
References
Syed F, Khosla S . Mechanism of sex steroid effects on bone. Biochem Biophys Res Commun 2005; 328( 3): 688–696.
Turner RT, Riggs BL, Spelsberg TC . Skeletal effects of estrogen. Endocr Rev 1994; 15( 3): 275–300.
Spelsberg TC, Subramaniam M, Riggs BL et al. The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol Endocrinol 1999; 13( 6): 819–828.
Richard DJ, Subramaniam M, Spelsberg TC . Molecular and cellular mechanisms of estrogen action on the skeleton. J Cell Biochem 1999; 75( S32/S33): 123–132.
Kameda T, Mano H, Yuasa T et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med 1997; 186( 4): 489–495.
Kassem M, Harris SA, Spelsberg TC et al. Estrogen inhibits interleukin-6 production and gene expression in a human osteoblastic cell line with high levels of estrogen receptors. J Bone Miner Res 1996; 11( 2): 193–199.
Oursler MJ, Cortese C, Keeting P et al. Modulation of transforming growth factor-β production in normal human osteoblast-like cells by 17β-estradiol and parathyroid hormone. Endocrinology 1991; 129( 6): 3313–3320.
Kousteni S, Chen JR, Bellido T et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 2002; 298( 5594): 843–846.
Boyce BF, Xing L . Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008; 473( 2): 139–146.
Hofbauer LC, Khosla S, Dunstan CR et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblast cells. Endocrinology 1999; 140( 9): 4367–4370.
Krum SA, Miranda-Carboni GA, Hauschka PV et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 2008; 27( 3): 535–545.
Zhou S, Turgeman G, Harris SE et al. Estrogens activate bone morphogenetic protein-2 gene transcription in mouse mesenchymal stem cells. Mol Endocrinol 2003; 17( 1): 56–66.
Eriksen EF, Colvard DS, Berg NJ et al. Evidence of estrogen receptors in normal human osteoblast-like cells. Science 1988; 241( 4861): 84–86.
Komm BS, Terpening CM, Benz DJ et al. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science 1988; 241( 4861): 81–84.
Qu Q, Perala-Heape M, Kapanen A et al. Estrogen enhances differentiation of osteoblasts in mouse bone marrow culture. Bone 1998; 22( 3): 201–209.
Tobias JH, Compston JE . Does estrogen stimulate osteoblast function in postmenopausal women? Bone 1999; 24( 2): 121–124.
Bradford PG, Gerace KV, Roland RL et al. Estrogen regulation of apoptosis in osteoblasts. Physiol Behav 2010; 99( 2): 181–185.
Palmer RM, Loveridge N, Thomson BM et al. Effects of a polyclonal antiserum to rat growth hormone on circulating insulin-like growth factor (IGF)-I and IGF-binding protein concentrations and the growth of muscle and bone. J Endocrinol 1994; 142: 85–91.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29( 9): e45.
Brazma A, Hingamp P, Quackenbush J et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet 2001; 29( 4): 365–371.
Ames MS, Hong S, Lee HR et al. Estrogen deficiency increases variability of tissue mineral density of alveolar bone surrounding teeth. Arch Oral Biol 2010; 55( 8): 599–605.
August M, Chung K, Chang Y et al. Influence of estrogen status on endosseous implant osseointegration. J Oral Maxillofac Surg 2001; 59( 11): 1285–1289.
Li YN, Wang D, Wang Z et al. The effect of 17β-estradiol on the osseointegration of dental implants in osteoporostic rats. Chin J Geriatr Dent 2012; 10( 3): 160–163.
Genetos DC, Geist DJ, Liu D et al. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblast. J Bone Miner Res 2005; 20( 1): 41–49.
Park JB . Combination of simvastatin and bone morphogenetic protein-2 enhances the differentiation of osteoblasts by regulating the expressions of phospho-Smad1/5/8. Exp Ther Med 2012; 4( 2): 303–306.
Katsuyama H, Arii M, Tomita M et al. Association between estrogen receptor alpha polymorphisms and equol production, and its relation to bone mass. Int J Mol Med 2009; 23( 6): 793–798.
Deng Y, Chen X, Hu YF et al. Effects of estrogen versus letrozole on chicken embryo frontal bone osteoblast. J Clin Rehabil Tissue Eng Res 2010; 14( 7): 1157–1161.
Shen Q, Zhu S, Hu J et al. Recombinant human bone morphogenetic protein-4 (BMP-4)-stimulated cell differentiation and bone formation within the expanding calvarial suture in rats. J Craniofac Surg 2009; 20( 5): 1561–1565.
Valverde-Franco G, Liu H, Davidson D et al. Defective bone mineralization and osteopenia in young adult FGFR3−/− mice. Hum Mol Genet 2004; 13( 3): 271–284.
Bradford PG, Gerace KV, Roland RL et al. Estrogen regulation of apoptosis in osteoblasts. Physiol Behav 2010; 99( 2): 181–185.
Kim S, Koga T, Isobe M et al. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev 2003; 17( 16): 1979–1991.
Tajima K, Takaishi H, Takito J et al. Inhibition of STAT1 accelerates bone fracture healing. J Orthop Res 2010; 28( 7): 937–941.
Woeckel VJ, Eijken M, van de Peppel J et al. IFNβ impairs extracellular matrix formation leading to inhibition of mineralization by effects in the early stage of human osteoblast differentiation. J Cell Physiol 2012; 227( 6): 2668–2676.
Yasuda H, Shima N, Nakagawa N et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 1998; 95( 7): 3597–3602.
Yasuda H, Shima N, Nakagawa N et al. Identity of osteoclastogenesis inhibitory factor(OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1998; 139( 3): 1329–1337.
Bucay N, Sarosi I, Dunstan CR et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998; 12( 9): 1260–1268.
Bord S, Ireland DC, Beavan SR et al. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003; 32( 2): 136–141.
Babij P, Zhao W, Small C et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 2003; 18( 6): 960–974.
Day TF, Guo X, Garrett-Beal L et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005; 8( 5): 739–750.
Hens JR, Wilson KM, Dann P et al. TOPGALl mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res 2005; 20( 7): 1103–1113.
Silkstone D, Hong H, Alman BA . Beta-catenin in the race to fracture repair: in it to Wnt. Nat Clin Pract Rheumatol 2008; 4( 8): 413–419.
Almeida M, Han L, Obrien CA et al. Classical genotropic versus kinase-initiated regulation of gene transcription by the estrogen receptor alpha. Endocrinology 2006; 147( 4): 1986–1996.
Kousteni S, Almeida M, Han L et al. Induction of osteoblast differentiation by selective activation of kinase-mediated actions of the estrogen receptor. Mol Cell Biol 2007; 27( 4): 1516–1530.
Holmen SL, Zylstra CR, Mukherjee A et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 2005; 280( 22): 21162–21168.
Gaur T, Lengner CJ, Hovhannisyan H et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 2005; 280( 39): 33132–33140.
Vandenberg AL, Sassoon DA . Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via van Gogh-like 2. Development 2009; 136( 9): 1559–1570.
Hakeda Y, Yoshino T, Natakani Y et al. Prostaglandin E2 stimulates DNA synthesis by a cyclic AMP-independent pathway in osteoblastic clone MC3T3-E1 cells. J Cell Physiol 1986; 128( 2): 155–161.
Xiao G, Jiang D, Thomas P et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem 2000; 275( 6): 4453–4459.
Wei P, Yuan GH, Yang MH et al. The Significance of the change of the gap junction protein connexin 43 expressed in osteoblasts and osteoclasts in ovaricetomized rats. J North Sichuan Med Coll 2006; 21( 4): 304–307.
Di WL, Lachelin GC, McGarrigle HH et al. Oestriol and oestradiol increase cell to cell communication and connexin43 protein expression in human myometrium. Mol Hum Reprod 2001; 7( 7): 671–679.
Chen CC, Lin CC, Lee TM . 17 Beta-estradiol decreases vulnerability to ventricular arrhythmias by preserving connexin43 protein in infracted rats. Eur J Pharmacol 2010; 629( 1/2/3): 73–81.
Ren J, Wang XH, Wang GC et al. 17β estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells. Bone 2013; 53( 2): 587–596.
Luo G, Hofmann C, Bronckers AL et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 1995; 9( 22): 2808–2820.
Ducy P, Zhang R, Geoffroy V et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997; 89( 5): 747–754.
Takuwa Y, Ohse C, Wang EA et al. Bone morphogenetic protein-2 stimulates alkaline phosphatase activity and collagen synthesis in cultured osteoblastic cells, MC3T3-E1. Biochem Biophys Res Commun 1991; 174( 1): 96–101.
Maliakal JC, Asahina I, Hauschka PV et al. Osteogenic protein-1 (BMP-7) inhibits cell proliferation and stimulates the expression of markers characteristic of osteoblast phenotype in rat osteosarcoma (17/2.8) cells. Growth Factors 1994; 11( 3): 227–234.
Li IW, Cheifetz S, McCulloch CA et al. Effects of osteogenic protein-1 (OP-1, BMP-7) on bone matrix protein expression by fetal rat calvarial cells are differentiation stage specific. J Cell Physiol 1996; 169( 1): 115–125.
Yeh LC, Adamo ML, Olson MS et al. Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology 1997; 138( 10): 4181–4190.
Bord S, Horner A, Hembry RM et al. Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles inskeletal development. Bone 1998; 23( 1): 7–12.
Breckon JJ, Papaioannou S, Kon LW et al. Stromelysin (MMP-3) synthesis is up-regulated in estrogen-deficient mouse osteoblasts in vivo and in vitro. J Bone Miner Res 1999; 14( 11): 1880–1890.
Dew G, Murphy G, Stanton H et al. Localisation of matrix metalloproteinases and TIMP-2 in resorbing mouse bone. Cell Tissue Res 2000; 299( 3): 385–394.
Kusano K, Miyaura C, Inada M et al. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mousecalvaria: association of MMP induction with bone resorption. Endocrinology 1998; 139( 3): 1338–1345.
Inoue K, Mikuni-Takagaki Y, Oikawa K et al. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem 2006; 281( 44): 33814–33824.
Apte SS, Fukai N, Beier DR et al. The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J Biol Chem 1997; 272( 41): 25511–25517.
Acknowledgements
This work was supported by grants from the Natural Science Fund (ZR2010HM035) of Shandong Province and from the Shandong Provincial Health Development Project Fund (2011WSB19002) in China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Shang, ZZ., Li, X., Sun, HQ. et al. Differentially expressed genes and signalling pathways are involved in mouse osteoblast-like MC3T3-E1 cells exposed to 17-β estradiol. Int J Oral Sci 6, 142–149 (2014). https://doi.org/10.1038/ijos.2014.2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijos.2014.2
Keywords
This article is cited by
-
Effects of pyrite bioleaching solution of Acidithiobacillus ferrooxidans on viability, differentiation and mineralization potentials of rat osteoblasts
Archives of Pharmacal Research (2015)