Abstract
A previous study demonstrated that alexidine has greater affinity for the major virulence factors of bacteria than chlorhexidine. The aim of this study was to compare the antimicrobial activity of 1% alexidine with that of 2% chlorhexidine using Enterococcus faecalis-infected dentin blocks. Sixty bovine dentin blocks were prepared and randomly divided into six groups of 10 each. E. faecalis was inoculated on 60 dentin blocks using the Luppens apparatus for 24 h and then the dentin blocks were soaked in 2% chlorhexidine or 1% alexidine solutions for 5 and 10 min, respectively. Sterile saline was used as a control. The antimicrobial efficacy was assessed by counting the number of bacteria adhering to the dentin surface and observing the degradation of bacterial shape or membrane rupture under a scanning electron microscope. Significantly fewer bacteria were observed in the 2% chlorhexidine- or 1% alexidine-soaked groups than in the control group (P<0.05). However, there was no significant difference in the number of bacteria adhering to the dentinal surface between the two experimental groups or between the two soaking time groups (P>0.05). Ruptured or antiseptic-attached bacteria were more frequently observed in the 10-min-soaked chlorhexidine and alexidine groups than in the 5-min-soaked chlorhexidine and alexidine groups. In conclusion, 10-min soaking with 1% alexidine or 2% chlorhexidine can be effective against E. faecalis infection.
Similar content being viewed by others
Introduction
Elimination of microbial contamination from the root canal system at the time of canal obturation is a prerequisite for successful outcomes in endodontic therapy.1 Bacteria in the root canal are present either as free-floating single cells or attached to each other or to the root canal walls to form a biofilm.2,3 Enterococcus faecalis, a Gram-positive and facultative anaerobic bacterium, is more likely to be found in persistent infections than in primary infections.4 The inherent ability of E. faecalis to adhere and invade dentinal tubules5,6 and form communities in an organized biofilm may contribute to bacterial resistance and persistence of infection after root canal treatment.7 Thus, many studies have been conducted on E. faecalis-infected dentin blocks to determine the antimicrobial activity of intracanal disinfectants.8,9 Mechanical instrumentation alone does not result in total or permanent reduction of bacteria from infected root canals10 and thus, requires the concomitant use of various antimicrobial irrigants in a sequential manner or in combination to enhance the antimicrobial effect.11
Chlorhexidine (CHX) digluconate is a bisguanide disinfectant that has antimicrobial substantive activity,12,13,14 and thus has been widely used as an auxiliary canal irrigant or a canal soaking agent against E. faecalis.15,16 A recent study also demonstrated that CHX attenuates the activity of E. faecalis lipoteichoic acid (LTA).17 However, CHX was shown to have no tissue solvent activity.15
Alexidine (ALX) is also a bisguanide disinfectant that has greater affinity for the major virulence factors such as bacterial lipopolysaccharide and LTA than CHX.18 It was also reported that the interaction of ALX and sodium hypochlorite did not form an insoluble precipitate known as para-chloroaniline.19 However, to date, its antimicrobial efficacy on E. faecalis biofilms is unknown. Therefore, the aim of this study was to compare the antimicrobial activity of 1% ALX with that of 2% CHX using E. faecalis-infected bovine dentin blocks. The hypothesis was that 1% ALX and 2% CHX would show no difference in antibacterial efficacy against E. faecalis.
Materials and methods
Preparation of dentin blocks
Bovine incisor teeth were extracted, and the roots were cleaned with scalers and 4% sodium hypochlorite (NaOCl) solution. The coronal and apical thirds of the roots were removed using a Microtome (Struers, Rodovre, Denmark). The middle thirds of the roots were split along the long axis. The specimens were immersed in 4% NaOCl for 5 min and then 17% ethylene diaminetetraacetic acid (EDTA) for 2 min to remove the smear layer, then washed with distilled water and sterilized by autoclaving.
E. faecalis inoculation
E. faecalis (ATCC 29212) inoculation was carried out using the apparatus reported by Luppens et al.20 The apparatus consisted of a vessel containing 10-fold-diluted brain–heart infusion (BHI; Difco Laboratories, Detroit, MI, USA), a pump (Masterflex pump system; Cole-Parmer Instrument Co., Chicago, IL, USA), a culture container (perfusion culture container 1301; Minucells and Minutissues, Bad Abbach, Germany), and a vessel for waste, all of which were connected with silicone tubes (Figure 1). Before inoculation, samples were placed in the culture container, and BHI was pumped through the system for 30 min. To inoculate E. faecalis, culture media (BHI) in the container was removed. The 24-h culture (15 mL) of E. faecalis (5.5×109 colony-forming units per mL) was then added to the container. After 30 min, the pump was restarted, and samples were allowed to develop a biofilm for 24 h in the presence of a constant nutrient flow. After the pump was stopped, the blocks were removed from the container and placed into the cell culture wells (1 dentin block per well) of a 24-well plate.
Preparation of antibacterial solutions
The dentin blocks were soaked in 1 mL of 2% CHX or 1% ALX for 5 and 10 min, respectively. A 2% solution of CHX was prepared by diluting a 20% solution of CHX (C 9394; Sigma-Aldrich, St Louis, MO, USA) with sterile distilled water. A 1% solution of ALX was prepared by dissolving ALX dihydrochloride powder (M68182628; Gentaur, Kampenhout, Belgium) in sterile distilled water. We tested 1% concentration of ALX solution because ALX with a concentration higher than 1% caused moderate cytotoxicity against human gingival fibroblasts in our preliminary study. Sterile saline was used as the control group.
Scanning electron microscopic observation and statistical analysis
After the aforementioned treatment, all dentin blocks were fixed in 2% glutaraldehyde at 4 °C and prepared for observation under a scanning electron microscope (SEM). The central beam of the SEM (Hitachi S-4700, Tokyo, Japan) was directed to the surface of each dentin block under 50 times magnification in order to observe the whole sample surface. Then, 10 areas of each specimen projected onto the screen were randomly selected and magnified 1 500 times. The number of bacteria observed within the grid was counted. To observe the shape of bacteria and their characteristics, areas of interest were photographed under 10 000 and 20 000 times magnification. The final results for the mean number of E. faecalis binding to dentin were obtained by calculating the mean scores of the 10 selected areas of each specimen.21 The data were analyzed using one-way ANOVA, and the comparison of means was conducted using the Tukey post hoc multiple comparison test. P value less than 0.05 was considered statistically significant.
Results
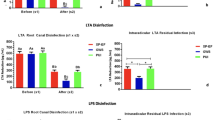
The numbers of bacteria counted in three different groups are summarized in Table 1. In the groups soaked with 2% CHX or 1% ALX for 5 min, significantly fewer bacterial cells were observed than in the saline (control) group (P=0.002), but there was no significant difference in the number of bacteria adhering to the dentinal surface between the two experimental groups (P=0.869). In the groups soaked with 2% CHX or 1% ALX for 10 min, the number of bacteria was significantly reduced compared with the control group (P=0.004). However, there was no statistically significant difference in the number of adhered bacteria between the ALX and CHX groups (P=0.674).
Under a SEM, the shape of the E. faecalis attached to the dentinal surface was normal in the group soaked with saline for 5 min (Figure 2a and 2b). On the contrary, E. faecalis with damaged (ruptured) membranes were often found in the 5-min-soaked ALX and CHX groups (Figure 2c–2f). No remarkable difference in bacterial shape could be seen between control groups soaked with saline for 5 or 10 min (Figures 2a, 2b, 3a and 3b). However, antiseptic particles were more frequently attached to the bacterial surface in the 10-min-soaked CHX and ALX groups (Figure 3c–3f) than the 5-min-soaked CHX and ALX groups (Figure 2c–2f). In particular, damaged or antiseptic-attached bacteria were frequently observed in the 10-min-soaked ALX group (Figure 3f).
SEM images of E. faecalis -infected dentin blocks in the 5-min-soaked groups. SEM images of E. faecalis-infected dentin blocks treated with saline show a large number of adhering bacteria (a, ×1 500) with intact bacterial membranes (b, ×20 000). The group treated with 2% CHX shows reduced numbers of adhering bacteria (c, ×1 500) and many lysed E. faecalis (d, ×10 000, white arrow). The group treated with ALX also shows fewer adhering bacteria (e, ×1 500) with damaged membranes (f, ×10 000, white arrow). ALX, alexidine; CHX, chlorhexidine; E. faecalis, Enterococcus faecalis; SEM, scanning electron microscope.
SEM images of E. faecalis -infected dentin blocks in the 10-min-soaked groups. SEM images of E. faecalis-infected dentin blocks treated with saline for 10 min show many adhering E. faecalis (a, ×1 500) with normal shape (b, ×20 000). The group soaked with 2% CHX shows fewer adhering bacteria (c, ×1 500) and CHX particles attached to bacterial membranes (d, ×20 000, white arrow). The group treated with 1% ALX also shows fewer adhering bacteria with abnormal shape (e, ×1 500) and ALX particles attached to bacterial membranes (f, ×20 000, white arrow). ALX, alexidine; CHX, chlorhexidine; E. faecalis, Enterococcus faecalis; SEM, scanning electron microscope.
Discussion
This study supports the hypothesis that 1% ALX and 2% CHX have no difference in antibacterial activity against E. faecalis. In the present study, 5-min soaking with 2% CHX or 1% ALX significantly reduced the number of bacteria adhering to the dentinal surface compared to the control group, but the antiseptic-attached bacteria were scarcely observed. On the contrary, in the 10-min soaking with CHX or ALX groups, antiseptic particles attached to the bacterial surface and abnormal bacterial shapes were commonly found, particularly in the ALX group. These findings imply two clinically important insights. One is that the antibacterial activity of ALX or CHX is due to the adherence of antiseptic particles to the bacterial cell wall. The other is that maximum adherence of antiseptic particles to the bacterial cell wall took longer than 5 min. The clinical implication of these findings is that ALX or CHX should be in direct contact with the infected dentinal surface for a prolonged time (>5 min) in order to achieve their maximum antibacterial effect against E. faecalis. In general, Gram-positive bacteria like E. faecalis are more sensitive to cations, because they are negatively charged. Chlorhexidine is a cationic bisguanide that seems to act by adhering onto the negatively charged phosphate groups of bacterial cell walls, causing leakage of intracellular components.22 At higher concentrations, as were used in this study, CHX has a bactericidal effect due to precipitation and/or coagulation of the cytoplasm, probably caused by protein cross-linking.23 ALX, similar to CHX, is a cationic bisguanide that induces lipid phase separation and domain formation at bacterial membranes.24 However, ALX has greater affinity for bacterial LTA than CHX.19 This characteristic might result in the formation of many ruptured (damaged) or antiseptic-attached bacteria in the 10-min-soaked ALX group. Furthermore, both irrigants have substantive antimicrobial activity.25 This can allow ALX to be applied as a supplementary final rinse before intracanal medication or a canal soaking agent before canal obturation, as well as a drug component of controlled release device.26
The inoculation system used in this study was the method utilized by Luppens et al.20 The Luppens apparatus has several advantages over previous methods suggested by Das et al.27 and Wright et al.28 First, the Luppens method counts bacterial cell number rather than determining the minimum inhibitory concentration of the antibacterial agent, which is less appropriate since growth-inhibited cells (e.g., E. faecalis) can still recover, regrow and reinfect the root canal system. Second, the Luppens method applies shear force and poor nutritional supply when creating the biofilm. The resulting biofilms with firmly attached bacteria more closely simulate the biofilms formed in the infected root canal system. Finally, the Luppens apparatus is simple and economical compared to previous methods such as the Robbins device.29
A recent study emphasized the importance of standardization of factors such as biofilm age when comparing the effectiveness of disinfecting agents against biofilm bacteria.30 Regarding this issue, a previous study assessed the efficacy of CHX and cetrimide in eradicating 1-day-old biofilms of E. faecalis.31 Another study reported that 1-day-old biofilms of E. faecalis provide a better comparison of the antimicrobial effectiveness of disinfectants than 3-day-old biofilms.32 The study revealed that 3-day-old biofilms showed a natural decrease in adhered bacteria with additional 1-day incubation despite the absence of an antibacterial agent. On the contrary, 1-day-old biofilms showed no change in the number of adhered bacteria with additional 1-day incubation. This implies that 3-day-old biofilms could have ‘natural’ loss of adhered bacteria not caused by an antibacterial irrigant. Considering this report, we used the Luppens apparatus to yield 1-day-old biofilms of E. faecalis, which might be a sufficient time period for obtaining adequate biofilm density.32,33,33
In the present study, the SEM was only used to observe the numbers or the degradation of bacterial shape or membrane rupture of bacteria attached on dentin substrate, but not their viability. Therefore, alternative viability evaluation such as LIVE/DEAD bacterial viability test with a confocal laser scanning microscope would be helpful in evaluation of the antimicrobial efficacy, in future study. Also, the present study did not include sodium hypochlorite as a positive control because its antimicrobial mode against E. faecalis is different from that of ALX and CHX. Sodium hypochlorite kills E. faecalis by the high alkaline pH34 but the ALX and CHX attach to the bacterial membrane surface, cause leakage of intracellular components and rupture bacterial membranes, which are clearly shown in the SEM observation of our study.
Although the present study showed that 1% ALX was effective against E. faecalis infection, we recommend ALX as a supplementary final rinse before intracanal medication or as canal soaking agent before canal obturation in persistently-infected/failed root canals, because ALX does not possess organic tissue-dissolving properties like sodium hypochlorite.35 In addition, ALX was studied to be a possible replacement of CHX in root canal irrigation, since the interaction of ALX and sodium hypochlorite did not form an insoluble precipitate known as para-chloroaniline.19 Therefore, strategies to treat E. faecalis-infected root canals may benefit from mechanical disruption of the multicellular bacterial structure by nickel–titanium instrumentation, dissolution of the extracellular polymeric substance by NaOCl irrigation, supplementary rinse with ALX before intracanal medication, calcium hydroxide medication, and canal soaking with ALX or CHX solution for 10 min before canal obturation. This treatment protocol may synergistically improve the success rate of endodontic treatment in E. faecalis-infected root canals.
Conclusion
Under the limitation of the present study, 1% ALX has a similar antibacterial effect to 2% CHX against E. faecalis, suggesting that both irrigants can be useful for a supplementary final rinse before intracanal medication or as canal soaking agents before canal obturation in E.faecalis-infected root canals.
References
Sjögren U, Figdor D, Persson S et al. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J 1997; 30( 5): 297–306.
Rucucci D, Siqueira JF Jr . Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod 2010; 36( 8): 1277–1288.
Haapasalo M, Endal U, Zandi H et al. Eradication of endodontic infection by instrumentation and irrigation solution. Endod Topics 2005; 10( 1): 77–102.
Siren EK, Haapasalo MPP, Ranta K et al. Microbial findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. Int Endod J 1997; 30( 2): 90–95.
Sedgley CM, Lennan SL, Appelbe OK . Survival of Enterococcus faecalis in root canals ex vivo. Int Endod J 2005; 38( 10): 735–742.
Chivatxaranukul P, Dashper SG, Messer HH . Dentinal tubule invasion and adherence by Enterococcus faecalis. Int Endod J 2008; 41( 10): 873–882.
Distel JW, Hatton JF, Gillespie MJ . Biofilm formation in medicated root canals. J Endod 2002; 28( 10): 689–693.
Giardino L, Ambu E, Savoldi E et al. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and Tetraclan against Enterococcus faecalis biofilm. J Endod 2007; 33( 7): 852–855.
Arias-Moliz MT, Ferrer-Luque CM, Espigares GarciaM et al. Enterococcus faecalis biofilms eradication by root canal irrigants. J Endod 2009; 35( 5): 711–714.
Byström A, Sundqvist G . Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res 1981; 89( 4): 321–328.
Zehnder M . Root canal irrigants. J Endod 2006; 32( 5): 389–398.
Parsons GJ, Patterson SS, Miller CH et al. Uptake and release of chlorhexidine by bovine pulp and dentin specimens and their subsequent acquisition of antibacterial properties. Oral Surg Oral Med Oral Pathol Oral Radiol 1980; 49( 5): 455–459.
Basrani B, Santos JM, Tjaderhane L et al. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94( 2): 240–245.
Baca P, Junco P, Arias-Moliz MT et al. Antimicrobial substantivity over time of chlorhexidine and cetrimide. J Endod 2012; 38( 7): 927–930.
Mohammadi Z, Abbott PV . The properties and applications of chlorhexidine in endodontics. Int Endod J 2009; 42( 4): 288–302.
Gomez BP, Ferraz CC, Vianna ME et al. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J 2001; 34( 6): 424–428.
Lee JK, Baik JE, Yun CH et al. Chlorhexidine gluconate attenuates the ability of lipoteichoic acid from Enterococcus faecalis to stimulate toll-like receptor 2. J Endod 2009; 35( 2): 212–215.
Zorko M, Jerala R . Alexidine and chlorhexidine bind to lipopolysaccharide and lipoteichoic acid and prevent cell activation by antibiotics. J Antimicrob Chemoth 2008; 62( 4): 730–737.
Kim HS, Zhu Q, Han SH et al. Chemical interaction of alexidine and sodium hypochlorite. J Endod 2012; 38( 1): 112–116.
Luppens SB, Reij MW, van der Heijden RW et al. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl Environ Microbiol 2002; 68( 9): 4194–4200.
Yang SE, Cha JH, Kim ES et al. Effect of smear layer and chlorhexidine treatment on the adhesion of Enterococcus faecalis to bovine dentin. J Endod 2006; 32( 7): 663–667.
Greenstein G, Berman C, Jaffin R . Chlorhexidine. An adjunct to periodontal therapy. J Periodontol 1986; 57( 6): 370–376.
Kontakiotis E, Nakou M, Georgopoulou M . In vitro study of the indirect action of calcium hydroxide on the anaerobic flora of the root canal. Int Endod J 1995; 28( 6): 285–289.
Baker PJ, Coburn RA, Genco RJ et al. Structural determinants of activity of chlorhexidine and alkyl bisbiguanides against the human oral flora. J Dent Res 1987; 66( 6): 1099–1106.
Roberts WR, Addy M . Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine: I. Effect on plaque accumulation and salivary bacteria. J Clin Periodontol 1981; 8( 3): 213–219.
Lee Y, Han SH, Hong SH et al. Antimicrobial efficacy of a polymeric chlorhexidine release device using in vitro model of Enterococcus faecalis infected dentinal tubule infection. J Endod 2008; 34( 7): 855–858.
Das JR, Bhakoo M, Jones MV et al. Changes in the biocide susceptibility of Staphylococcus epidermidis and Escherichia coli cells associated with rapid attachment to plastic surfaces. J Appl Microbiol 1998; 84( 5): 852–858.
Wright TL, Ellen RP, Lacroix JM et al. Effects of metronidazole on Porphyromonas gingivalis biofilms. J Periodontal Res 1997; 32( 5): 473–477.
Adams JL, McLean RJ . Impact of rpoS deletion on Escherichia coli biofilms. Appl Environ Microbiol 1999; 65( 9): 4285–4287.
Shen Y, Stojicic S, Haapasalo M . Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod 2011; 37( 5): 657–661.
Arias-Moliz M, Ferrer-Luque C, Gonzalez-Rodriquez M et al. Eradiation of Enteroccus faecalis biofilms by cetrimide and chlorhexidine. J Endod 2010; 36( 1): 87–90.
Lima K, Fava L, Siqueira J . Susceptibilities of Enterococcus faecalis biofilms to some antimicrobial medications. J Endod 2001; 27( 10): 616–619.
Persoon IF, Hoogenkamp MA, Bury A et al. Effect of vanadium chloroperoxidase on Enterococcus faecalis biofilms. J Endod 2012; 38( 1): 72–74.
Portenier I, Waltimo TMT, Haapasalo M . Enterococcus faecalis—the root canal survivor and ‘star’ in post-treatment disease. Endod Topics 2003; 6( 1): 135–169.
Naenni N, Thoma K, Zehnder M . Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod 2004; 30( 11): 785–787.
Acknowledgements
This study was supported by the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (MEST) (No.2009-0086835, 2011-0014231, 2012-0008693: Drs KY Kum, SH Han and SW Chang), South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kim, HS., Woo Chang, S., Baek, SH. et al. Antimicrobial effect of alexidine and chlorhexidine against Enterococcus faecalis infection. Int J Oral Sci 5, 26–31 (2013). https://doi.org/10.1038/ijos.2013.11
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijos.2013.11
Keywords
This article is cited by
-
Rational design of EDTA-incorporated nanoflowers as novel and effective endodontic disinfection against biofilms
Odontology (2024)
-
Comparison of antibacterial activity of alexidine alone or as a final irrigant with sodium hypochlorite and chlorhexidine
BDJ Open (2018)
-
Bridging the gap between traditional cell cultures and bioreactors applied in regenerative medicine: practical experiences with the MINUSHEET perfusion culture system
Cytotechnology (2016)
-
Antimicrobial activity of alexidine, chlorhexidine and cetrimide against Streptococcus mutans biofilm
Annals of Clinical Microbiology and Antimicrobials (2014)
-
Residual activity of cetrimide and chlorhexidine on Enterococcus faecalis-infected root canals
International Journal of Oral Science (2014)