Abstract
Background:
Offspring of obese mothers have increased risk of developing obesity and related short- and long-term disease. The cause is multifactorial and may partly be explained by the unfavorable intrauterine environment. Intervention during pregnancy leading to a healthier lifestyle among obese may alter this.
Objective:
To assess the effect of lifestyle intervention on markers of maternal metabolism and inflammation in ‘the TOP (Treatment of Obese Pregnant Women) study’, a randomized controlled trial.
Methods:
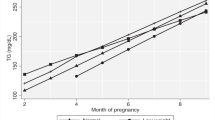
In the TOP-study 425 participants with body mass index ⩾30 kg/m2 were randomized to intervention with dietary advices and physical activity assessed by pedometer (PA+D), physical activity assessed by pedometer (PA) or control (C). Of 389 participants completing the study 376 had available blood samples. Serum was analyzed for insulin, c-peptide, lipid profile, leptin, high-sensitivity CRP (hsCRP) and Soluble urokinase Plasminogen Activator Receptor (suPAR), in week 18–20 and 28–30, and simultaneously a 2-h oral glucose-tolerance-test was performed. Diet was assessed in gestational week 11–14 and 36–37 using a validated 360-item Food Frequency Questionnaire.
Results:
Median levels of hsCRP in gestational week 28–30 were lower in each of the intervention groups (8.3 mg/l in PA+D group, P=0.03; and 8.8 mg/l in PA group, P=0.02) versus the control group (11.5 mg/l). Obtaining 11 000 steps per day as aimed for resulted in a 21% lower hsCRP compared to non-compliant women. Women reporting high carbohydrate intake had around 30% higher hsCRP concentrations in late gestation than women reporting the lowest intake. There were no differences in lipid profile or any of the metabolic markers in gestational week 28–30 when comparing the intervention and control groups.
Conclusions:
Lifestyle intervention in obese women can reduce hsCRP representing a marker of inflammation during pregnancy. The effect may partly be mediated by more physical activity and partly by changes in intake of carbohydrates and the glycaemic load.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nelson SM, Matthews P, Poston L . Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010; 16: 255–275.
Ovesen P, Rasmussen S, Kesmodel U . Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 2011; 118 (2 Pt 1): 305–312.
Catalano PM, Ehrenberg HM . The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006; 113: 1126–1133.
Schellong K, Schulz S, Harder T, Plagemann A . Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012; 7: e47776.
Huda SS, Brodie LE, Sattar N . Obesity in pregnancy: prevalence and metabolic consequences. Semin Fetal Neonatal Med 2010; 15: 70–76.
Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002.
Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012; 308: 1150–1159.
Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N . Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 2002; 87: 4231–4237.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334: 292–295.
Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR . Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab 2007; 92: 969–975.
Haupt TH, Kallemose T, Ladelund S, Rasmussen LJ, Thorball CW, Andersen O et al. Risk factors associated with serum levels of the inflammatory biomarker soluble urokinase plasminogen activator receptor in a general population. Biomark Insights 2014; 9: 91–100.
Toldi G, Biro E, Szalay B, Stenczer B, Molvarec A, Rigo J et al. Soluble urokinase plasminogen activator receptor (suPAR) levels in healthy pregnancy and preeclampsia. Clin Chem Lab Med 2011; 49: 1873–1876.
Haedersdal S, Salvig JD, Aabye M, Thorball CW, Ruhwald M, Ladelund S et al. Inflammatory markers in the second trimester prior to clinical onset of preeclampsia, intrauterine growth restriction, and spontaneous preterm birth. Inflammation 2013; 36: 907–913.
Lyngbaek S, Sehestedt T, Marott JL, Hansen TW, Olsen MH, Andersen O et al. CRP and suPAR are differently related to anthropometry and subclinical organ damage. Int J Cardiol 2013; 167: 781–785.
Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppesen JL . suPAR: a new biomarker for cardiovascular disease? Can J Cardiol 2015; 31: 1293–1302.
El Khouly NI, Sanad ZF, Saleh SA, Shabana AA, Elhalaby AF, Badr EE . Value of first-trimester serum lipid profile in early prediction of preeclampsia and its severity: a prospective cohort study. Hypertens Pregnancy 2016; 35: 73–81.
Spracklen CN, Smith CJ, Saftlas AF, Robinson JG, Ryckman KK . Maternal hyperlipidemia and the risk of preeclampsia: a meta-analysis. Am J Epidemiol 2014; 180: 346–358.
Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012; 344: e2088.
Moses RG, Luebcke M, Davis WS, Coleman KJ, Tapsell LC, Petocz P et al. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am J Clin Nutr 2006; 84: 807–812.
Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015; 3: 767–777.
Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jorgensen JS . The LiP (Lifestyle in Pregnancy) Study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011; 34: 2502–2507.
Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM . Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 2012; 345: e5605.
Renault KM, Norgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol 2014; 210: 134–139.
Olsen S, Dragsted L, Hansen H, Milman N, Ovesen L, Michaelsen K et al Scientific evidence underpinning dietary recommendations in connection with pregnancy Ernæringsrådet. Danish National Nutrition Council: Søborg, Denmark, 2005. Report No. 38..
Renault K, Norgaard K, Andreasen KR, Secher NJ, Nilas L . Physical activity during pregnancy in obese and normal-weight women as assessed by pedometer. Acta Obstet Gynecol Scand 2010; 89: 956–961.
Olsen SF, Mikkelsen TB, Knudsen VK, Orozova-Bekkevold I, Halldorsson TI, Strom M et al. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr Perinat Epidemiol 2007; 21: 76–86.
Mikkelsen TB, Osler M, Olsen SF . Validity of protein, retinol, folic acid and n-3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutr 2006; 9: 771–778.
Volund A . Conversion of insulin units to SI units. Am J Clin Nutr 1993; 58: 714–715.
Khoury J, Henriksen T, Christophersen B, Tonstad S . Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. Am J Obstet Gynecol 2005; 193: 1292–1301.
Renault KM, Carlsen EM, Norgaard K, Nilas L, Pryds O, Secher NJ et al. Intake of Sweets, Snacks and Soft Drinks Predicts Weight Gain in Obese Pregnant Women: Detailed Analysis of the Results of a Randomised Controlled Trial. PLoS ONE 2015; 10: e0133041.
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC . Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr 2006; 84: 1489–1497.
Esposito K, Marfella R, Ciotola M, Di PC, Giugliano F, Giugliano G et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004; 292: 1440–1446.
Buyken AE, Goletzke J, Joslowski G, Felbick A, Cheng G, Herder C et al. Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr 2014; 99: 813–833.
Hammonds TL, Gathright EC, Goldstein CM, Penn MS, Hughes JW . Effects of exercise on c-reactive protein in healthy patients and in patients with heart disease: A meta-analysis. Heart Lung 2016; 45: 273–282.
Scholl TO, Chen X, Goldberg GS, Khusial PR, Stein TP . Maternal diet, C-reactive protein, and the outcome of pregnancy. J Am Coll Nutr 2011; 30: 233–240.
Syngelaki A, Visser GH, Krithinakis K, Wright A, Nicolaides KH . First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism 2016; 65: 131–137.
Danielsen I, Granstrom C, Haldorsson T, Rytter D, Hammer BB, Henriksen TB et al. Dietary glycemic index during pregnancy is associated with biomarkers of the metabolic syndrome in offspring at age 20 years. PLoS ONE 2013; 8: e64887.
Sen S, Rifas-Shiman SL, Shivappa N, Wirth MD, Hebert JR, Gold DR et al. Dietary inflammatory potential during pregnancy is associated with lower fetal growth and breastfeeding failure: results from project viva. J Nutr 2016; 146: 728–736.
Lowe LP, Metzger BE, Lowe WL Jr., Dyer AR, McDade TW, McIntyre HD . Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab 2010; 95: 5427–5434.
Retnakaran R, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B et al. Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. CMAJ 2012; 184: 1353–1360.
Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014; 348: g1285.
Oostdam N, van Poppel MN, Wouters MG, Eekhoff EM, Bekedam DJ, Kuchenbecker WK et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG 2012; 119: 1098–1107.
Walsh JM, Mahony RM, Culliton M, Foley ME, McAuliffe FM . Impact of a low glycemic index diet in pregnancy on markers of maternal and fetal metabolism and inflammation. Reprod Sci 2014; 21: 1378–1381.
Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A . A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008; 32: 495–501.
Vinter CA, Jorgensen JS, Ovesen P, Beck-Nielsen H, Skytthe A, Jensen DM . Metabolic effects of lifestyle intervention in obese pregnant women. Results from the randomized controlled trial 'Lifestyle in Pregnancy' (LiP). Diabet Med 2014; 31: 1323–1330.
Ray JG, Diamond P, Singh G, Bell CM . Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG 2006; 113: 379–386.
Catalano P, deMouzon SH . Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond) 2015; 39: 642–649.
Acknowledgements
‘Sygekassernes Helsefond’, ‘Brødrene Hartmann Fonden’, and the Danish Council for Strategic Research (09-067124, Centre for Fetal Programming) supported this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Renault, K., Carlsen, E., Hædersdal, S. et al. Impact of lifestyle intervention for obese women during pregnancy on maternal metabolic and inflammatory markers. Int J Obes 41, 598–605 (2017). https://doi.org/10.1038/ijo.2017.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.9
This article is cited by
-
Association between food environments and fetal growth in pregnant Brazilian women
BMC Pregnancy and Childbirth (2023)
-
Maternal leucocyte trajectory across pregnancy associated with offspring’s growth
Pediatric Research (2022)
-
Moderate-intensity continuous training and high-intensity interval training improve cognition, and BDNF levels of middle-aged overweight men
Metabolic Brain Disease (2022)
-
Physical activity during pregnancy and association with changes in fat mass and adipokines in women of normal-weight or with obesity
Scientific Reports (2021)
-
Supporting women of childbearing age in the prevention and treatment of overweight and obesity: a scoping review of randomized control trials of behavioral interventions
BMC Women's Health (2020)