Abstract
Background/Objectives:
Exposure to metabolic stress has been suggested to influence the susceptibility to metabolic disorders in offspring according to epidemiological and animal studies. Nevertheless, molecular mechanisms remain unclear. We investigated impacts of diet-induced paternal obesity on metabolic phenotypes in offspring and its underlying molecular mechanism.
Subjects/Methods:
Male founder mice (F0), fed with control diet (CD) or high-fat diet (HFD), were mated with CD-fed females. F1 progenies were mated with outbred mice to generate F2 mice. All offspring were maintained on CD. Metabolic phenotypes, metabolism-related gene expression and endoplasmic reticulum (ER) stress markers were measured in serum or relevant tissues of F2 mice. DNA methylation in sperm and testis of the founder and in the liver of F2 mice was investigated.
Results:
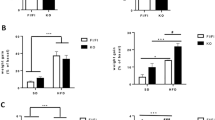
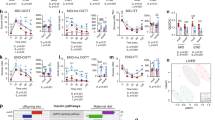
Male founder obesity, instigated by HFD, led to glucose dysregulation transmitted down to F2. We found that F2 males to HFD founders were overweight and had a high fasting glucose relative to F2 to CD founders. F2 females to HFD founders, in contrast, had a reduced bodyweight relative to F2 to CD founders and exhibited an early onset of impaired glucose homeostasis. The sex-specific difference was associated with distinct transcriptional patterns in metabolism-related organs, showing altered hepatic glycolysis and decreased adipose Glucose transporter 4 (Glut4) in males and increased gluconeogenesis and lipid synthesis in females. Furthermore, the changes in females were linked to hepatic ER stress, leading to suppressed insulin signaling and non-obese hyperglycemic phenotypes. DNA methylation analysis revealed that the Nr1h3 locus was sensitive to HFD at founder germ cells and the alteration was also detected in the liver of F2 female.
Conclusion:
Our findings demonstrate that male founder obesity influences impaired glucose regulation in F2 progeny possibly via ER stress in a sex-specific manner and it is, in part, contributed by altered DNA methylation at the Nr1h3 locus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jauch-Chara K, Schmoller A, Oltmanns KM . Impaired glucose tolerance in healthy men with low body weight. Nutr J 2011; 10: 16.
Færch K, Witte DR, Tabák AG, Perreault L, Herder C, Brunner EJ et al. Trajectories of cardiometabolic risk factors before diagnosis of three subtypes of type 2 diabetes: a post-hoc analysis of the longitudinal Whitehall II cohort study. Lancet Diabetes Endocrinol 2013; 1: 43–51.
Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, Arriola L et al. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes 2014; 63: 4378–4387.
Ravelli AC, van der Meulen JH, Osmond C, Barker DJ, Bleker OP . Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 1999; 70: 811–816.
Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 2006; 14: 159–166.
Simmons RA, Templeton LJ, Gertz SJ . Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 2001; 50: 2279–2286.
Bringhenti I, Moraes-Teixeira JA, Cunha MR, Ornellas F, Mandarim-de-Lacerda CA, Aguila MB . Maternal obesity during the preconception and early life periods alters pancreatic development in early and adult life in male mouse offspring. PLoS ONE 2013; 8: e55711.
Dunn GA, Bale TL . Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 2009; 150: 4999–5009.
Ng S-F, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ . Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010; 467: 963–966.
Fullston T, Teague EMCO, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J 2013; 27: 4226–4243.
Bligh EG, Dyer WJ . A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–917.
Fullston T, McPherson NO, Owens JA, Kang WX, Sandeman LY, Lane M . Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet. Physiol Rep 2015; 3: e12336.
de Castro Barbosa T, Ingerslev LR, Alm P, Versteyhe S, Massart J, Rasmussen M et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab 2015; 5: 184–197.
Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y et al. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab 2012; 16: 825–832.
Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U . Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab 2015; 26: 193–200.
Muoio DM, Newgard CB . Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9: 193–205.
Özcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Özdelen E et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461.
Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI . Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE 2013; 8: e54059.
Zhang X, Tang S, Zhang Q, Shao W, Han X, Wang Y et al. Endoplasmic reticulum stress mediates JNK-dependent IRS-1 serine phosphorylation and results in Tau hyperphosphorylation in amyloid beta oligomer-treated PC12 cells and primary neurons. Gene 2016; 587: 183–193.
Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A et al. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol 2009; 29: 5070–5083.
Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K et al. A central role for JNK in obesity and insulin resistance. Nature 2002; 420: 333–336.
Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA et al. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem 2004; 279: 45803–45809.
Martínez D, Pentinat T, Ribó S, Daviaud C, Bloks VW, Cebrià J et al. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered lxra DNA methylation. Cell Metab 2014; 19: 941–951.
Grandjean V, Fourré S, De Abreu DAF, Derieppe M-A, Remy J-J, Rassoulzadegan M . RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep 2015; 5: 18193.
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016; 351: 397–400.
Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E et al. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 2014; 345: 1255903.
Huypens P, Sass S, Wu M, Dyckhoff D, Tschöp M, Theis F et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 2016; 48: 497–499.
Rong X, Albert CJ, Hong C, Duerr MA, Chamberlain BT, Tarling EJ et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab 2013; 18: 685–697.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF 2016R1D1A1A02937546 to YJP and 2014R1A1A2058964 to AML). JHP acknowledges funding from Health Fellowship Foundation, and MYC and YY were supported by Brain Korea 21 plus project (22A20130012143). We thank Ewha Laboratory Animal Genomic Center (Ewha Womans University) for help with animal care, and the Asan Medical Center (Seoul, Republic of Korea).
Author contributions
YJP conceived the study, and YJP and AML designed the experiments; JHP and YY performed all animal experiments; JHP, YY, MC, JL and AML did the biochemical and molecular analyses; JHP and YJP interpreted the data; JHP, YJP and AML discussed and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Park, J., Yoo, Y., Cho, M. et al. Diet-induced obesity leads to metabolic dysregulation in offspring via endoplasmic reticulum stress in a sex-specific manner. Int J Obes 42, 244–251 (2018). https://doi.org/10.1038/ijo.2017.203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.203
This article is cited by
-
Diet-induced obesity in animal models: points to consider and influence on metabolic markers
Diabetology & Metabolic Syndrome (2021)
-
Paternal Nongenetic Intergenerational Transmission of Metabolic Disease Risk
Current Diabetes Reports (2019)