Abstract
Background:
Obesity is known as an epidemic worldwide because of consumption of westernized high-fat diets and one of the major risk factors of hypertension. Histone deacetylases (HDACs) control gene expression by regulating histone/non-histone protein deacetylation. HDAC inhibitors exert anti-cancer and anti-inflammatory effects and play a protective role in cardiovascular diseases. In the present study, we tested the effect of an FDA-approved pan-HDAC inhibitor valproic acid (VPA) on high-fat diet (HFD)-induced hypertension in mice. Furthermore, we examined the mechanism of VPA-induced prevention of hypertension.
Methods:
Nine-week-old male C57BL/6 mice were fed either a normal diet (ND) or HFD. When the HFD group reached a pre-hypertensive phase (130–140 mm Hg systolic blood pressure), VPA was administered for 6 days (300 mg kg−1 per day). Body weights and blood pressure (BP), expression of renin-angiotensin system (RAS) components and HDAC1 were determined. The direct role of HDAC1 in the expression of RAS components was investigated using gene silencing.
Results:
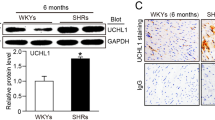
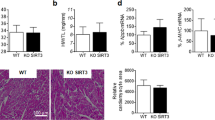
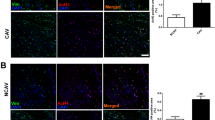
HFD accelerated the increase in body weight from 22.4±1.3 to 31.9±3.0 compared to in the ND group from 22.7±0.9 to 26.0±1.7 (P=0.0134 ND vs HFD), systolic BP from 118.5±5.7 to 145.0±3.0 (P<0.001), and diastolic BP from 91.0±13.6 to 121.0±5.0 (P=0.006); BP was not altered in the ND group. HFD increased RAS components and HDAC1 in the kidneys as well as leptin in the plasma. VPA administration prevented the progression of hypertension and inhibited the increase in expression of HDAC1 and RAS components. VPA did not affect plasma leptin level. Knockdown of HDAC1 in MDCK cells decreased the expression of angiotensinogen and type 1 angiotensin II receptor.
Conclusions:
VPA prevented HFD-induced hypertension by downregulating angiotensin II and its receptor via inhibition of HDAC1, offering a novel therapeutic option for HFD-induced hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
WHOObesity and overweighthttp://www.who.int/mediacentre/factsheets/fs311/en/ 2016.
Kelly T, Yang W, Chen CS, Reynolds K, He J . Global burden of obesity in 2005 and projections to 2030. Int J Obes 2008; 32: 1431–1437.
Pi-Sunyer X . The medical risks of obesity. Postgrad Med 2009; 121: 21–33.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME . Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015; 116: 991–1006.
Montani JP, Antic V, Yang Z, Dulloo A . Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord 2002; 26 (Suppl 2): S28–S38.
Robles RG, Villa E, Santirso R, Martínez J, Ruilope LM, Cuesta c et al. Effects of captopril on sympathetic activity, lipid and carbohydrate metabolism in a model of obesity-induced hypertension in dogs. Am J Hypertens 1993; 6: 1009–1015.
Hall JE . The kidney, hypertension, and obesity. Hypertension (Dallas, Tex.: 1979) 2003; 41: 625–633.
Grassi G, Seravalle G, Dell'Oro R, Trevano FQ, Bombelli M, Scopelliti F et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens 2003; 21: 1761–1769.
Dorresteijn JA, Schrover IM, Visseren FL, Scheffer PG, Oey PL et al. Differential effects of renin-angiotensin-aldosterone system inhibition, sympathoinhibition and diuretic therapy on endothelial function and blood pressure in obesity-related hypertension: a double-blind, placebo-controlled cross-over trial. J Hypertens 2013; 31: 393–403.
Redon J, Cifkova R, Laurent S, Nilsson P, Narkiewicz K, Erdine S et al. The metabolic syndrome in hypertension: European society of hypertension position statement. J Hypertens 2008; 26: 1891–1900.
Thaker V, Patel K . Recent advances in pharmacotherapy of acute coronary syndrome. Int J Basic Clin Pharmacol 2017; 5: 1695–1703.
Viera AJ . Resistant hypertension. J Am Board Fam Med 2012; 25: 487–495.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520.
Arguelles AO, Meruvu S, Bowman JD, Choudhury M . Are epigenetic drugs for diabetes and obesity at our door step? Drug Discov Today 2016; 21: 499–509.
Tang J, Yan H, Zhuang S . Histone deacetylases as targets for treatment of multiple diseases. Clin Sci 2013; 124: 651–662.
Wang Y, Miao X, Liu Y, Li F, Liu Q, Sun J et al. Dysregulation of histone acetyltransferases and deacetylases in cardiovascular diseases. Oxid Med Cell Longev 2014; 2014: 641979.
Eom GH, Kook H . Posttranslational modifications of histone deacetylases: implications for cardiovascular diseases. Pharmacol Ther 2014; 143: 168–180.
FDAValproate Informationhttp://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm192645.htm 2016.
NIHHIV Drugs in Developmenthttps://chemdb.niaid.nih.gov/DrugDevelopmentHIV.aspx 2016.
Chateauvieux S, Morceau F, Dicato M, Diederich M . Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol 2010; 2010: 18http://www.ncbi.nlm.nih.gov/pubmed/20798865.
Shabason JE, Tofilon PJ, Camphausen K . HDAC inhibitors in cancer care. Oncology 2010; 24: 180–185.
Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension 2010; 56: 437–444.
Li RF, Cao SS, Fang WJ, Song Y, Luo XT, Wang HY et al. Roles of HDAC2 and HDAC8 in Cardiac Remodeling in Renovascular Hypertensive Rats and the Effects of Valproic Acid Sodium. Pharmacology 2016; 99: 27–39.
Rajeshwari T, Raja B, Manivannan J, Silambarasan T, Dhanalakshmi T . Valproic acid prevents the deregulation of lipid metabolism and renal renin-angiotensin system in L-NAME induced nitric oxide deficient hypertensive rats. Environ Toxicol Pharmacol 2014; 37: 936–945.
Kim JI . High fat diet confers vascular hyper-contractility against angiotensin II through upregulation of MLCK and CPI-17. Korean J Physiol Pharmacol 2017; 21: 99–106.
Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV . Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 2004; 279: 52282–52292.
Seok YM, Lee HA, Park KM, Hwangbo M-H, Kim IK . Lysine deacetylase inhibition attenuates hypertension and is accompanied by acetylation of mineralocorticoid receptor instead of histone acetylation in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 2016; 389: 799–808.
Coffman TM . The inextricable role of the kidney in hypertension. J Clin Invest 2014; 124: 2341–2347.
Zhao L et al. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 2012; 126: 455–467.
Morimatsu Y et al. Development and characterization of an animal model of severe pulmonary arterial hypertension. J Vasc Res 2012; 49: 33–42.
Rondelet B et al. Bosentan for the prevention of overcirculation-induced experimental pulmonary arterial hypertension. Circulation 2003; 107: 1329–1335.
Wu TH, Kuo HC, Lin IC, Chien SJ, Huang LT, Tain YL et al. Melatonin prevents neonatal dexamethasone induced programmed hypertension: histone deacetylase inhibition. J Steroid Biochem Mol Biol 2014; 144: Pt B,253–259.
Soltani Z, Washco V, Morse S, Reisin E . The impacts of obesity on the cardiovascular and renal systems: cascade of events and therapeutic approaches. Curr Hypertens Rep 2015; 17: 7.
Lan B, Hayama E, Kawaguchi N, Furutani Y, Nakanishi T . Therapeutic efficacy of valproic acid in a combined monocrotaline and chronic hypoxia rat model of severe pulmonary hypertension. PLoS ONE 2015; 10: e0117211.
Brenner BM, C M, de Zeeuw D, Keane WF, Mitch WE, Parving HH et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869.
Parish RC, Miller LJ . Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Safety 1992; 7: 14–31.
Struhl K . Histone acetylation and transcriptional regulatory mechanisms. Genes Dev 1998; 12: 599–606.
Chen Y, Cai S, Wang J, Xu M . Valproic acid-induced histone acetylation suppresses CYP19 gene expression and inhibits the growth and survival of endometrial stromal cells. Int J Mol Med 2015; 36: 725–732.
Qiao L, Schaack J, Shao J . Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology 2006; 147: 865–874.
Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 2011; 145: 607–621.
Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD et al. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell 2001; 8: 1243–1254.
Lee HA, Lee DY, Cho HM, Kim SY, Iwasaki Y, Kim IK et al. Histone deacetylase inhibition attenuates transcriptional activity of mineralocorticoid receptor through its acetylation and prevents development of hypertension. Circ Res 2013; 112: 1004–1012.
Seok YM, Lee HA, Park KM, Hwangbo MH, Kim IK . Lysine deacetylase inhibition attenuates hypertension and is accompanied by acetylation of mineralocorticoid receptor instead of histone acetylation in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 2016; 389: 799–808.
Wolden-Hanson T, Gidal BE, Atkinson RL . Evaluation of a rat model of valproate-induced obesity. Pharmacotherapy 1998; 18: 1075–1081.
Coble JP, Cassell MD, Davis DR, Grobe JL, Sigmund CD . Activation of the renin-angiotensin system, specifically in the subfornical organ is sufficient to induce fluid intake. Am J Physiol 2014; 307: R376–R386.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant (MSIP No. 2014R1A5A2010008 and NRF-2017R1D1A1B03032729) funded by the Korean Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, J., Park, S., Kwon, T. et al. Role of the histone deacetylase inhibitor valproic acid in high-fat diet-induced hypertension via inhibition of HDAC1/angiotensin II axis. Int J Obes 41, 1702–1709 (2017). https://doi.org/10.1038/ijo.2017.166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.166
This article is cited by
-
Valproate decreases transgenerationally blood pressure by affecting thyrotropin-releasing hormone promoter DNA methylation and gene expression in spontaneously hypertensive rat
Molecular and Cellular Biochemistry (2024)
-
Kidney and epigenetic mechanisms of salt-sensitive hypertension
Nature Reviews Nephrology (2021)
-
An anticonvulsive drug, valproic acid (valproate), has effects on the biosynthesis of fatty acids and polyketides in microorganisms
Scientific Reports (2020)
-
Associations of IL1RAP and IL1RL1 gene polymorphisms with obesity and inflammation mediators
Inflammation Research (2020)
-
Histone deacetylase inhibitor CG200745 ameliorates high-fat diet-induced hypertension via inhibition of angiotensin II production
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)