Abstract
Background:
Both circadian disruption and timing of feeding have important roles in the development of metabolic disease. Despite growing acceptance that the timing of food consumption has long-term impact on metabolic homeostasis, little is known regarding the immediate influence on whole body metabolism, or the mechanisms involved. We aimed to examine the acute effects of time-of-day-dependent high fat feeding on whole body substrate metabolism and metabolic plasticity, and to determine the potential contribution of the adipocyte circadian clock.
Methods:
Mice were fed a regimen of 4-h meal at the beginning and end of the dark (waking) cycle, separated by 4 h of fasting. Daily experimental conditions consisted of either an early very high fat or high fat (EVHF or EHF, 60 or 45% kcals from fat, respectively) or late (LVHF or LHF) meal, paired with a low fat (LF, 10% kcals from fat) meal. Metabolic parameters, glucose tolerance, body fat composition and weight were assessed. To determine the role of the adipocyte circadian clock, an aP2-CLOCK mutant (ACM) mouse model was used.
Results:
Mice in the EVHF or EHF groups showed a 13.2 or 8.84 higher percentage of caloric intake from fat and had a 0.013 or 0.026 lower daily average respiratory exchange ratio, respectively, compared with mice eating the opposite feeding regime. Changes in glucose tolerance, body fat composition and weight were not significant at the end of the 9-day restricted feeding period. ACM mice did not exhibit different metabolic responses to the feeding regimes compared with wild-type littermates. Circadian clock disruption did not influence the short-term response to timed feeding.
Conclusions:
Both the total fat composition of diet and the timing of fat intake may differentially mediate the effect of timed feeding on substrate metabolism, but may not induce acute changes in metabolic flexibility.
Similar content being viewed by others
Introduction
Daily rhythms of multiple metabolic parameters are regulated by circadian clocks, a transcriptional mechanism found in virtually every cell of an organism. At the core of this molecular clock mechanism are Bmal1 (brain and muscle arnt-like protein 1) and Clock (circadian locomotor output cycles kaput). BMAL1 and CLOCK proteins bind together to form a heterodimer, which activates transcription of several genes, including the core negative regulating elements, Per (period) and Cry (cryptochrome). This negative feedback system results not only in rhythmic expression of clock component genes, but also downstream targets, including genes, involved in multiple metabolic pathways, feeding behavior and energy balance.1
Forced dyssynchrony between normal sleep/wake and feeding/fasting cycles through restriction of food access has been shown to alter glucose tolerance and influence weight gain in rodents fed both high fat (HF) and normal diets. Mice with food access restricted to the light phase were shown to gain more weight2, 3 and exhibit insulin resistance4 compared to mice with food access restricted to the dark phase. Conversely, HF food access restricted to 4 h of the light phase between zeitgeber time (ZT) 4 and 8 (ZT0: lights on) was associated with lower caloric intake, greater insulin sensitivity and lower total cholesterol levels compared with ad libitum HF feeding.5 Compared with ad libitum fed mice, mice with restricted HF chow (61% calories from fat) availability to 8 h during the dark phase (ZT13-21) gained less weight and had improved glucose tolerance over 18 weeks.6 These beneficial effects were also apparent in mice fed 8-h (ZT13-ZT21) restricted normal chow (13% calories from fat), suggesting that timing of food intake might improve metabolism under diverse nutritional challenges.6 We previously have shown that restricting consumption of low fat (LF) (10% calories from fat) food to the beginning of the dark phase coupled with HF (45% calories from fat) food at the end of the dark phase over 12 weeks leads to increased weight gain, adiposity and glucose intolerance.7 Similarly, a 6-h advance or delay of usual meal time affected daily rhythms of blood glucose and triglyceride levels in C57BL/6J male mice.4 Collectively, these observations suggest that both total daily caloric intake and timing of feeding are important for metabolic homeostasis.
Although it is becoming clear that the timing of nutrient intake has an important role in homeostasis, it is not known how quickly manipulation of food intake timing perturbs metabolic processes. The current study investigates the acute effects of distinct time-of-day-dependent feeding strategies on metabolic plasticity, adiposity and glucose tolerance. To examine the possible contribution of cell autonomous clocks, we used an aP2-CLOCK mutant (ACM) mouse model;8 this animal model expresses a mutant CLOCKΔ19 protein9 driven by the aP2 promoter. Thus, the clock mechanism is disrupted in cells in which aP2 is active, including adipocytes and macrophages. Mice harboring the CLOCKΔ19 mutation in a germline manner develop obesity, as well as exhibit increased blood serum triglycerides, cholesterol, glucose and leptin levels when on a standard chow diet compared with their wild-type (WT) counterparts.10 The ACM mouse has been shown to present a similar phenotype to the global CLOCK mutant mouse, gaining more weight and fat mass as they age compared with WT mice.8 The purpose of this study was to examine the acute effects of time-of-day-dependent HF feeding on whole body substrate metabolism and metabolic plasticity, and to determine the potential contribution of the adipocyte circadian clock.
Materials and Methods
Animals
Male ACM and WT mice on an FVB/N background were housed under a 12h:12h light:dark. Mice were given water and chow ad libitum unless otherwise specified. Mice were housed in standard micro-isolator cages, before initiation of feeding protocols (during which time mice were housed on wire-bottom cages to prevent consumption of bedding and feces). The animal protocol followed was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham (Birmingham, AL, USA).
Rodent diets
Mice were fed a LF diet (10% calories from fat, Research Diets, New Brunswick, NJ, USA; D12450B), a HF diet (45% calories from fat, Research Diets, New Brunswick, NJ, USA; D12451) or a very high fat (VHF) diet (60% calories from fat, Research Diets; D12492). All diets were matched for protein content (20% calories from protein) and source of dietary macro and micronutrients.
Restricted time-of-day feeding
To test the effects of circadian disruption and altered feeding strategies on glucose tolerance and metabolic plasticity, 20-week-old ACM and WT mice (n=5–7, Figure 1a) initially weaned onto standard chow at 3 weeks were singly housed within a Comprehensive Laboratory Animal Monitor System (CLAMS, Columbus Instruments Inc., Columbus, OH, USA) under a 12 h:12 h light:dark cycle (lights on at 0800 h; ZT0). The CLAMS measured food intake, energy expenditure and respiratory exchange ratio (RER) in a non-invasive manner. The CLAMS was configured with triple axis detection of animal motion using IR photocell technology for activity monitoring. When the animal traverses a cage, it breaks series of IR beams in sequence. These interruptions were scored as total physical activity. Repeated interruptions of the same IR beam did not contribute to the scores of total activity. Animals were housed in the CLAMS for a total of 13 days, consisting of 4 days of baseline measures in which animals were acclimated to the CLAMS and provided the LF meals in an ad libitum manner, followed by 9 days of restricted feeding per the regimen described below.
Sample size of the study under a Feeding × Genotype factorial design (a) and feeding regimes for timed feeding (b). In (b), mice were either fed (A) HF diet (45% calories from fat) during the first 4 h, followed by control diet (10% calories from fat) during the last 4 h of the active phase (EHF), (B) VHF diet (60% calories from fat) during the first 4 h, followed by VHF diet during the last 4 h of the active phase (EVHF), (C) Control diet during the first 4 h, followed by HF diet during the last 4 h of the active phase (EVHF) or (D) Control diet during the first 4 h, followed by VHF diet during the last 4 h of the active phase (LVHF). Diets were matched for protein content (20% calories from protein).
Animals were fed HF, VHF or LF protein-matched chow in a time-of-day-dependent manner, designed to simulate 'meals' of different compositions presented at distinct times during the awake/active phase. The awake/active phase was divided into three distinct 4-h time periods (Time Period 1 (TP1, ZT12–ZT16), Time Period 2 (TP2, ZT16–ZT20) and Time Period 3 (TP3, ZT20–ZT24), respectively, Figure 1b). Four-hour meals at the beginning and end of the waking period were separated by 4 h of food restriction. Early high fat (EHF) and early very high fat (EVHF) groups were given a HF or VHF 'meal' during the first 4 h of the dark phase (that is, sleep/wake transition, TP1) followed by no food for 4 h (that is, TP2) and a LF 'meal' during the last 4 h of the dark phase (that is, TP3). Late high fat (LHF) and late very high fat (LVHF) groups were given a LF 'meal' at the onset of the dark phase (that is, TP1) followed by no food for 4 h (that is, TP2) and a HF or VHF 'meal' during the last 4 h of the dark phase (that is, TP3). Access to food was restricted during the inactive (lights on) period (ZT0–ZT12). Feeding regimes were enforced by a computer-controlled CLAMS.
Body composition
Body composition was determined in mice using the Lunar PIXImus Densitometer (GE Medical Systems, Madison, WI, USA). Mice were sedated with ketamine/xylaxine (80 and 16 mg kg−1, respectively).
Glucose tolerance test
Immediately following the 9-day feeding regimes, mice were singly housed in wire-bottom cages and fasted for 10 h. Tails were nicked and baseline plasma glucose measurements were taken using a Freestyle glucometer (Therasense, Alameda, CA, USA). Animals were injected intraperitoneally with 10% d-glucose (0.01 ml g−1). Plasma glucose levels were measured at 15, 30, 60, 90 and 120 min after injection. Tails were cauterized after each test. The area under the curve (AUC) was calculated from glucose tolerance tests results to quantify glucose responses.
Statistical analysis
All analyses were conducted using R (R Core Team 2014, R Foundation for Statistical Computing, Vienna, Austria). For the CLAMS data, the first 48 h of the baseline period and the first 24 h of restricted feeding period were considered acclimation periods to the experimental conditions and eliminated from data analysis. CLAMS data reduced to four 15 min intervals per hour: 0–15 min, 16–30 min, 31–45 min and 46–59 min. The data were then collapsed by experimental hours, creating mean values for the metabolic parameters and summarized by six 4-h intervals per day. We used the linear mixed-effect model to investigate the main effects of diet, genotype and time. The likelihood ratio test was used to calculate the P-value from the chi-squared distribution with one degree of freedom.
Main effects of diet and genotype on glucose tolerance, body composition and weight were tested using the two-way ANOVA model. Significance was set at P<0.05. Average values and standard error of mean (s.e.m.) are shown in the figures.
Results
We reported previously that meals ingested on waking have dramatic effects on energy homeostasis and metabolic plasticity following a 3-month intervention.7 As a follow-up to our previous research, here we investigated the acute effects of timed feeding within a shorter intervention period (that is, 9 days). Two different HF composition diets were used to determine whether the timing of a HF and/or a VHF diet impact whole body metabolism in a similar manner.
To test the effects of adipocyte circadian disruption on metabolic parameters, we examined the effects of genotype in both linear mixed-effect and ANOVA models. No significant differences were observed between ACM and WT mice in this acute experiment; therefore, all animals were included in analyses, and the main effects of diet and time were evaluated in the following analyses adjusting genotype as a covariate based on a Feeding × Genotype factorial design.
To examine the acute effects of time-of-day-dependent HF feeding on energy balance, we utilized the CLAMS. As anticipated, EVHF and EHF mice consumed more fat-derived calories from daily energy intake compared with their counterparts during TP1, which was reversed during TP3 (Figures 2a and e). No significant difference was observed for the average daily calorie intake during the 9 days of restricted timed feeding (EHF vs LHF, 15.16±0.38 kcal day−1 vs 14.34±0.42 kcal day−1; EVHF vs LVHF, 13.95±0.22 kcal day−1 vs 14.31±0.42 kcal day−1; Figures 2b and f). Despite identical daily total caloric intake, during the 9 days of feeding, mice in the EVHF and EHF diet groups consumed more fat-derived calories from daily energy intake compare with LHF and LVHF diet groups, respectively (EHF vs LHF, 33.71±0.81% vs 24.87±1.14%; EVHF vs LVHF 44.15±0.84% vs 30.63±0.84%; Figures 2c and g), which is primarily due to larger food intake during TP1.
Mice fed a HF or a VHF diet were divided into two distinct feeding groups, as depicted in Figure 1. The effects of these feeding regimes on caloric intake, divided by zeitgeber hour (a and e), average daily calorie intake (kcal day−1; b and f), average percent daily intake of fat (c and g) and average percent daily intake of carbohydrate (d and h) were determined. Data are shown as mean±s.e.m. (n=11 EHF, n=11 LHF, n=12 EVHF and n=13 LVHF). *P<0.05 **P<0.01 ***P<0.001.
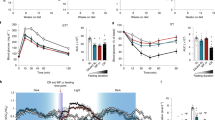
RER was utilized as an indicator of which fuel is being primarily metabolized to provide energy; a value of 0.7 indicates nearly complete dependence on fatty acid metabolism, while 1.0 indicates primary dependence on carbohydrate metabolism. As anticipated, indirect calorimetry revealed distinct 24-h patterns of RER between EVHF and LVHF mice as well as EHF and LHF mice (Figures 3a and d). In particular, EVHF mice experienced a significantly lower value of average daily RER compared with LVHF mice, indicating higher dependence on fatty acid oxidation on average (0.848±0.002 vs 0.876±0.002; Figure 3e). However, while mean estimates for RER were lower in EHF compared with LHF groups, this difference was not statistically significant (0.887±0.003 vs 0.900±0.003; Figure 3b).
The effects of distinct feeding regimes on respiratory quotient. Changes in RER throughout baseline and restricted timed feeding (a and d). Average daily values of RER (b and e). Average values of RER by 4-h zeitgeber interval (c and f). Data are shown as mean±s.e.m. (n=11 EHF, n=11 LHF, n=12 EVHF and n=13 LVHF). #The main effect of feeding regimes on RER during the inactive/light phase. **P<0.01, ***P<0.001.
We next assessed RER patterns with a narrower time window. For TP1 and TP2, there was a significant difference in RER values between EVHF and LVHF mice (TP1, 0.887±0.001 vs 1.102±0.002; TP2, 0.835±0.001 vs 1.005±0.002) as well as EHF and LHF mice (TP1, 0.956±0.002 vs 1.029±0.002; TP2, 0.915±0.002 vs 1.004±0.003; Figures 3c and f). EVHF and EHF mice maintained lower values of RER throughout the 4-h early meal and the 4-h fasting period compared with LVHF and LHF mice, suggesting an extended period of fatty acid oxidation. Nevertheless, within the 9-day period of timed feeding, animals remained responsive to dietary composition during TP3, with lowered RER for the LVHF and LHF groups in response to the late fat meals. Interestingly, the average RER values of LVHF and LHF mice across the 12 h of the light phase were significantly lower than those of their counterparts (EHF vs LHF, 0.822±0.001 vs 0.801±0.001; EVHF vs LVHF, 0.809±0.001 vs 0.773±0.007; Figures 3c and f).
To investigate how metabolic flexibility changed in response to timed feeding, we tested whether RER values were driven or 'fixed' by the early meal type throughout the entire awake/dark phase as time advanced in spite of a 4-h fast, as we had observed in our previous work.7 In this experiment, we did not observe any trend toward fixation of RER values. In this acute experiment, LVHF and LHF mice consistently demonstrated a decreasing trend in RER during the TP2 fasting period, which continued to decrease during HF consumption in TP3 (Figure 3). We examined whether this downward trend leveled out, but no significant change was detected within the 9- day experimental period.
To examine variability in RER values in the timed feeding regimen, we analyzed the differences in the peak values of RER during bouts of feeding for similar diets. Peak RER value during the LF meal was significantly higher (P<0.001) for both LVHF (1.02±0.06) and LHF mice (1.03±0.07) compared with EVHF (0.89±0.08) and EHF mice (0.95±0.08), respectively, suggesting a more pronounced shift toward carbohydrate utilization during the LF meal. In addition, the dynamic range of RER values was significantly smaller (P<0.001) in both EVHF (0.22±0.03) and EHF (0.28±0.04) groups, compared with the LVHF (0.33±0.04) and LHF (LHF 0.33±0.04) groups, indicating a more stable metabolism favored toward lipid utilization.
We next investigated energy expenditure in the experimental groups (Figure 4). LVHF mice exhibited a higher energy expenditure compared with EVHF mice throughout the 24-h time period (10.57±0.18 kcal day−1 vs 9.97±0.12 kcal day−1). Energy expenditure was also significantly higher during the early meal and 4 h lights-off fasting period for LVHF mice, as well as for the first 8 h of the light phase. Nevertheless, no significant differences were observed in physical activity for either HF or VHF groups (Figure 4).
The effects of distinct feeding regimes, divided by 4-h zeitgeber interval, on daily energy expenditure (a and b) and physical activity (e and f) for EHF vs LHF. The effects of distinct feeding regimes, divided by 4-h zeitgeber interval, on daily energy expenditure (c and d) and physical activity (g and h) for EVHF vs LVHF. Data are shown as mean±s.e.m. (n=11 EHF, n=11 LHF, n=12 EVHF and n=13 LVHF). *P<0.05, **P<0.01.
For body composition and glucose tolerance data, no significant differences in weight and body fat composition were observed with a short-term timed feeding (Figures 5 and 6).
The effects of distinct feeding regimes on glucose tolerance for EHF vs LHF (a) and EVHF vs LVHF (b). Plasma glucose levels were measured at 15, 30, 60, 90 and 120 min after intraperitoneal injection of 10% d-glucose (0.01 ml g−1). Data are shown as mean±s.e.m. (n=11 EHF, n=11 LHF, n=12 EVHF and n=13 LVHF).
Discussion
In this study, we observed a significant effect of time-of-day-dependent restricted feeding on substrate metabolism. EVHF and EHF mice were shown to have lower RER values during both the initial HF feeding period and the 4-h fast between feeding periods, indicating that they continue to mostly oxidize fatty acids upon waking compared with their counterparts who primarily oxidize carbohydrates, similar to results shown by Bray et al.7 LVHF and LHF mice were observed to have lower RER values during the light phase fast, possibly due to the HF meal at the end of their waking period accelerating the shift into fatty acid oxidation associated with an extended fasting period (ZT0–ZT12). In the short term, the last meal before sleeping seems to have the greatest influence an energy metabolism during the sleeping/fasting period. Intriguingly, EVHF mice demonstrated a significantly lower value of average daily RER compared with LVHF mice, while this difference was not observed between EHF and LHF groups. These observations may suggest that both the total fat content and the timing of fat intake impact substrate metabolism, and that these perturbations take place acutely following a transition to timed macronutrient intake.
One of the main purposes of the present study was to investigate the acute impact of time restricted feeding on metabolic flexibility, utilizing a typical human meal pattern. We previously have shown that metabolic plasticity is almost completely lost in the mice who consume a high-carbohydrate waking meal, independent of total energy intake.7 These mice demonstrated a ‘fixed’ pattern of metabolism toward carbohydrate usage, as RER values remained close to a value of 1 throughout the entire awake/dark period, despite a 4-h fast and a HF meal later in the waking period. However, it has remained unknown whether this time of feeding effect on metabolic flexibility is acute. Herein, we observed that mice fed a LH/high-carbohydrate waking meal do not experience acute detriments to metabolic flexibility with a short-term intervention, suggesting that loss of metabolic flexibility associated with a high-carbohydrate waking meal occurs with time. LVHF and LHF mice showed a consistent downward trend in RER values after having a VHF or HF diet, similar to EHF and EVHF mice. Similarly, the LVHF and LHF mice experience a decrease in RER during the TP2 fasting period. We further investigated a change in the slope of this decreasing trend across the days to determine whether metabolic flexibility was declining, but statistical analysis displayed no sign of change throughout the entire intervention period. Taking into account the marginal RER difference in the inactive/light phase, LVHF and LHF mice still appear to maintain metabolic plasticity by favoring fatty acid oxidation during TP3 and light phase within the 9- day feeding period. This result provides evidence that although timing of food intake affects metabolic pathways associated with nutritional physiology, long-term exposure is required to produce significant alterations in metabolic plasticity.
Meal timing and time restricted feeding have been shown previously to affect metabolism in mice and humans. Skipping breakfast is associated with increased risk of type 2 diabetes in humans,15 and consuming only one meal at the beginning of the active phase was associated with increased weight gain in mice.16 Restricted feeding to the light phase led to increased weight gain and insulin resistance in mice.2, 3, 4 Restricting HF chow to the dark phase resulted in less weight gain and improved glucose tolerance.6 A possible mechanism behind increased weight gain due to eating during the inactive phase may be related to dyssynchrony between the rhythmic expression of leptin and timing of feeding.17
To date, the mechanisms that mediate the effects of food on the circadian system are not yet clear.18 One potential mechanism is the zeitgeber effects of food on peripheral circadian clocks. Under normal light–dark condition with regular sleep timing, daily timed single carbohydrate-rich meals in human had a dramatic effect on the circadian phase position of core body temperature and heart rate without significantly shifting the dim-light melatonin onset.19 This result supports the observation that food is likely a zeitgeber for food-entrainable peripheral oscillation, but not the central oscillator.19 In line with this finding, Kuroda et al.20 demonstrated that the amount of food and the interval between feeding time markedly influence the phase of peripheral clock, even under the normal light–dark cycle. In this regard, we hypothesized that the peripheral adipocyte circadian clock has an important role in response of circadian system by food. Zvonic et al.21 previously demonstrated that circadian rhythmicity is apparent in both brown and white adipocyte, suggesting that the circadian clock within adipose tissue may have substantial effects on global metabolism and energy balance.
To investigate the function of circadian clock in adipose tissue, we introduced a mouse model with a mutant Clock gene driven by the aP2 promoter. This mouse model shows circadian disruption in adipose tissue but does not exhibit full body circadian disruption, allowing for a more detailed examination of function of peripheral clock within adipocyte.22 Although we did not observe a significant result for body composition and glucose tolerance data in either WT or mutant mice, 9 days of programmed feeding may not be sufficient to induce a meaningful difference in these measures. Therefore, studies with longer duration of time restricted feeding may be required to better define the mechanism of timed feeding effect on metabolic outcomes.
Based on our observations of metabolic flexibility and RER, a potential mechanism for long-term alterations in energy balance may also be through fluctuations in glucocorticoids. Previous studies have shown that glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators.11 Glucocorticoids are also thought to be important for energy balance because meal composition and timing affect secretion rhythmicity of glucocorticoids,12 and conversely, glucocorticoids impact on the motivation to eat-high sweet, fatty acid food.13 Although the effects of glucocorticoids on fatty acid metabolism are less well understood than those on glucose metabolism, glucocorticoids are believed to influence fatty acid oxidation by controlling both the uptake and turnover of fatty acids in adipose tissue, and eventually impact on the pathogenesis of the complications of obesity and the metabolic syndrome.14 We did not observe a weight gain difference between groups in this acute experiment, but the LHF meal promoted more weight gain as well as impaired the ability to adjust metabolism toward fat usage with long-term programmed feeding.7
Unexpectedly, LVHF mice exhibited higher daily energy expenditure than EVHF mice in contrast to insignificant difference in physical activity. Our study points to differences in energy metabolism that may independent of physical activity and potentially influenced by the timing of macronutrient intake. Current evidence suggests that meal composition may be a factor for altering energy expenditure,15, 16, 17 but only a limited number of studies have been conducted to figure out the role of meal timing on energy expenditure,18, 19, 20 and findings are still inconclusive. Recent studies18, 19 have indicated that meal timing may affect energy expenditure. Future study should evaluate how meal timing and composition influence energy influence and physical activity as a function of the circadian cycle.
Limitations of the study include a focus on dietary fat and carbohydrate and do not address the influence of dietary protein or fiber in energy balance and satiety, although both diets were matched for protein and fiber content. Additional mechanistic insight can be gained through measures of circadian and blood parameters, such as hormones, core body temperature, free fatty acid levels and fatty acid gene expression.
In conclusion, a short-term timed feeding appears to affect substrate metabolism, which may ultimately lead to long-term metabolic energy imbalance. Longer term studies are needed to identify the time course of the loss of metabolic flexibility associated with early LF/LHF feeding.
References
Gimble JM, Sutton GM, Ptitsyn AA, Floyd ZE, Bunnell BA . Circadian rhythms in adipose tissue: an update. Curr Opin Clin Nutr Metab Care 2011; 14: 554–561.
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW . Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009; 17: 2100–2102.
Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME . Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes (Lond) 2013; 37: 843–852.
Yoon J-A, Han D-H, Noh J-Y, Kim M-H, Son GH, Kim K et al. Meal time shift disturbs circadian rhythmicity along with metabolic and behavioral alterations in mice. PLoS One 2012; 7: e44053.
Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O . Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J 2012; 26: 3493–3502.
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong Eric A, Gill S et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 2012; 15: 848–860.
Bray MS, Tsai J-Y, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O et al. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes 2010; 34: 1589–1598.
Bray MS, Young ME . The role of cell-specific circadian clocks in metabolism and disease. Obes Rev 2009; 10: 6–13.
Vitaterna MH, King DP, Chang A-M, Kornhauser JM, Lowrey PL, McDonald JD et al. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science 1994; 264: 719–725.
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005; 308: 1043–1045.
Le Minh N . Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 2001; 20: 7128–7136.
So AYL, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ . Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA 2009; 106: 17582–17587.
Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF . Minireview: glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology 2004; 145: 2633–2638.
Macfarlane DP, Forbes S, Walker BR . Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol 2008; 197: 189–204.
Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB . Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr 2012; 95: 1182–1189.
Fuse Y, Hirao A, Kuroda H, Otsuka M, Tahara Y, Shibata S . Differential roles of breakfast only (one meal per day) and a bigger breakfast with a small dinner (two meals per day) in mice fed a high-fat diet with regard to induced obesity and lipid metabolism. J Circadian Rhythms 2012; 10: 4.
Arble DM, Vitaterna MH, Turek FW . Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS One 2011; 6: e25079.
Johnston JD . Physiological responses to food intake throughout the day. Nutr Res Rev 2014. FirstView 27: 1–12.
Kräuchi K, Cajochen C, Werth E, Wirz-Justice A . Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J Biol Rhythms 2002; 17: 364–376.
Kuroda H, Tahara Y, Saito K, Ohnishi N, Kubo Y, Seo Y et al. Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Sci Rep 2012; 2: 711.
Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 2006; 55: 962–970.
Birky TL, Bray MS . Understanding circadian gene function: animal models of tissue-specific circadian disruption. IUBMB Life 2014; 66: 34–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Joo, J., Cox, C., Kindred, E. et al. The acute effects of time-of-day-dependent high fat feeding on whole body metabolic flexibility in mice. Int J Obes 40, 1444–1451 (2016). https://doi.org/10.1038/ijo.2016.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.80