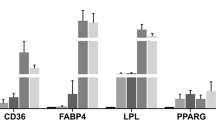
Abstract
Background/Objectives:
Consumption of fat-rich foods is associated with obesity and related alterations. However, there is a group of individuals, the metabolically obese normal-weight (MONW) subjects, who present normal body weight but have metabolic features characteristic of the obese status, including fat deposition in critical tissues such as liver, recognized as a major cause for the promotion of metabolic diseases. Our aim was to better understand metabolic alterations present in liver of MONW rats applying whole genome transcriptome analysis.
Methods:
Wistar rats were chronically fed a high-fat diet isocaloric relative to Control animals to avoid the hyperphagia and overweight and to mimic MONW features. Liver transcriptome analysis of both groups was performed.
Results:
Sustained intake of an isocaloric high-fat diet had a deep impact on the liver transcriptome, mainly affecting lipid metabolism. Although serum cholesterol levels were not affected, circulating triacylglycerols were lower, and metabolic adaptations at gene expression level indicated adaptation toward handling the increased fat content of the diet, an increased triacylglycerol and cholesterol deposition in liver of MONW rats was observed. Moreover, gene expression pointed to increased risk of liver injury. One of the top upregulated genes in this tissue was Krt23, a marker of hepatic disease in humans that was also increased at the protein level.
Conclusion:
Long-term intake of a high-fat diet, even in the absence of overweight/obesity or increase in classical blood risk biomarkers, promotes a molecular environment leading to hepatic lipid accumulation and increasing the risk of suffering from hepatic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Moreno LA, Gottrand F, Huybrechts I, Ruiz JR, Gonzalez-Gross M, DeHenauw S et al. Nutrition and lifestyle in european adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Adv Nutr 2014; 5: 615S–623S.
Diethelm K, Huybrechts I, Moreno L, De Henauw S, Manios Y, Beghin L et al. Nutrient intake of European adolescents: results of the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2014; 17: 486–497.
Bel-Serrat S, Mouratidou T, Huybrechts I, Labayen I, Cuenca-Garcia M, Palacios G et al. Associations between macronutrient intake and serum lipid profile depend on body fat in European adolescents: the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Br J Nutr 2014; 112: 2049–2059.
Labayen I, Ruiz JR, Ortega FB, Huybrechts I, Rodriguez G, Jimenez-Pavon D et al. High fat diets are associated with higher abdominal adiposity regardless of physical activity in adolescents; the HELENA study. Clin Nutr 2013; 33: 859–866.
Caterson ID, Gill TP . Obesity: epidemiology and possible prevention. Best Pract Res Clin Endocrinol Metab 2002; 16: 595–610.
Yajnik CS, Yudkin JS . The Y-Y paradox. Lancet 2004; 363: 163.
Lee SH, Ha HS, Park YJ, Lee JH, Yim HW, Yoon KH et al. Identifying metabolically obese but normal-weight (MONW) individuals in a nondiabetic Korean population: the Chungju Metabolic disease Cohort (CMC) study. Clin Endocrinol (Oxf) 2011; 75: 475–481.
Lopez-Miranda J, Perez-Martinez P . It is time to define metabolically obese but normal-weight (MONW) individuals. Clin Endocrinol 2013; 79: 314–315.
Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S . The metabolically obese, normal-weight individual revisited. Diabetes 1998; 47: 699–713.
Molero-Conejo E, Morales LM, Fernandez V, Raleigh X, Gomez ME, Semprun-Fereira M et al. Lean adolescents with increased risk for metabolic syndrome. Arch Latinoam Nutr 2003; 53: 39–46.
Dvorak RV, DeNino WF, Ades PA, Poehlman ET . Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes 1999; 48: 2210–2214.
Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB . The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003; 163: 427–436.
Conus F, Rabasa-Lhoret R, Peronnet F . Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab 2007; 32: 4–12.
Thomas EL, Parkinson JR, Frost GS, Goldstone AP, Dore CJ, McCarthy JP et al. The missing risk: MRI and MRS phenotyping of abdominal adiposity and ectopic fat. Obesity 2012; 20: 76–87.
Arsenault BJ, Beaumont EP, Despres JP, Larose E . Mapping body fat distribution: a key step towards the identification of the vulnerable patient? Ann Med 2012; 44: 758–772.
Despres JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387–1395.
Koo SH . Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol 2013; 19: 210–215.
Choi J, Se-Young O, Lee D, Tak S, Hong M, Park SM et al. Characteristics of diet patterns in metabolically obese, normal weight adults (Korean National Health and Nutrition Examination Survey III, 2005). Nutr Metab Cardiovasc Dis 2012; 22: 567–574.
Cao L, Liu X, Cao H, Lv Q, Tong N . Modified high-sucrose diet-induced abdominally obese and normal-weight rats developed high plasma free fatty acid and insulin resistance. Oxid Med Cell Longev 2012; 2012: 374346.
Rahati S, Shahraki M, Arjomand G, Shahraki T . Food pattern, lifestyle and diabetes mellitus. Int J High Risk Behav Addict 2014; 3: e8725.
Palou M, Priego T, Sanchez J, Torrens JM, Palou A, Pico C . Moderate caloric restriction in lactating rats protects offspring against obesity and insulin resistance in later life. Endocrinology 2010; 151: 1030–1041.
Diaz-Rua R, Garcia-Ruiz E, Caimari A, Palou A, Oliver P . Sustained exposure to diets with an unbalanced macronutrient proportion alters key genes involved in energy homeostasis and obesity-related metabolic parameters in rats. Food Funct 2014; 5: 3117–3131.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Caimari A, Oliver P, Palou A . Adipose triglyceride lipase expression and fasting regulation are differently affected by cold exposure in adipose tissues of lean and obese Zucker rats. J Nutr Biochem 2012; 23: 1041–1050.
Seifter S, Dayton S, Novic B, Muntwyler E . The estimation of glycogen with the anthrone reagent. Arch Biochem 1950; 25: 191–200.
Folch J, Lees M, Sloane Stanley GH . A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497–509.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254.
Garcia-Ruiz E, Reynes B, Diaz-Rua R, Ceresi E, Oliver P, Palou A . The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int J Obes (Lond) 2015; 39: 1619–1629.
van Schothorst EM, Pagmantidis V, de Boer VC, Hesketh J, Keijer J . Assessment of reducing RNA input for Agilent oligo microarrays. Anal Biochem 2007; 363: 315–317.
Duivenvoorde LP, van Schothorst EM, Bunschoten A, Keijer J . Dietary restriction of mice on a high-fat diet induces substrate efficiency and improves metabolic health. J Mol Endocrinol 2011; 47: 81–97.
Diaz-Rua R, Keijer J, Caimari A, van Schothorst EM, Palou A, Oliver P . Peripheral blood mononuclear cells as a source to detect markers of homeostatic alterations caused by the intake of diets with an unbalanced macronutrient composition. J Nutr Biochem 2015; 26: 398–407.
Hochberg Y, Benjamini Y . More powerful procedures for multiple significance testing. Stat Med 1990; 9: 811–818.
Hsu SM, Raine L, Fanger H . Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981; 29: 577–580.
Starmann J, Falth M, Spindelbock W, Lanz KL, Lackner C, Zatloukal K et al. Gene expression profiling unravels cancer-related hepatic molecular signatures in steatohepatitis but not in steatosis. PloS One 2012; 7: e46584.
Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005; 81: 341–354.
Otero YF, Stafford JM, McGuinness OP . Pathway-selective insulin resistance and metabolic disease: the importance of nutrient flux. J Biol Chem 2014; 289: 20462–20469.
Yoon HJ, Cha BS . Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol 2014; 6: 800–811.
Kim S, Sohn I, Ahn JI, Lee KH, Lee YS, Lee YS . Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene 2004; 340: 99–109.
Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 2007; 292: E1724–E1739.
Rao MS, Reddy JK . Peroxisomal beta-oxidation and steatohepatitis. Semin Liver Dis 2001; 21: 43–55.
Hsu MH, Savas U, Griffin KJ, Johnson EF . Human cytochrome p450 family 4 enzymes: function, genetic variation and regulation. Drug Metab Rev 2007; 39: 515–538.
Reddy JK, Rao MS . Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 2006; 290: G852–G858.
Hardwick JP, Osei-Hyiaman D, Wiland H, Abdelmegeed MA, Song BJ . PPAR/RXR regulation of fatty acid metabolism and fatty acid omega-hydroxylase (CYP4) isozymes: implications for prevention of lipotoxicity in fatty liver disease. PPAR Res 2009; 2009: 952734.
Cotter DG, Schugar RC, Crawford PA . Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol 2013; 304: H1060–H1076.
Selfridge JE, Wilkins HM, E L, Carl SM, Koppel S, Funk E et al. Effect of one month duration ketogenic and non-ketogenic high fat diets on mouse brain bioenergetic infrastructure. J Bioenerg Biomembr 2014; 47: 1–11.
Abdel-Hamid NM, Nazmy MH, Abdel-Bakey AI . Polyol profile as an early diagnostic and prognostic marker in natural product chemoprevention of hepatocellular carcinoma in diabetic rats. Diabetes Res Clin Pract 2011; 92: 228–237.
Renaud HJ, Cui JY, Lu H, Klaassen CD . Effect of diet on expression of genes involved in lipid metabolism, oxidative stress, and inflammation in mouse liver-insights into mechanisms of hepatic steatosis. PLoS One 2014; 9: e88584.
VerHague MA, Cheng D, Weinberg RB, Shelness GS . Apolipoprotein A-IV expression in mouse liver enhances triglyceride secretion and reduces hepatic lipid content by promoting very low density lipoprotein particle expansion. Arterioscler Thromb Vasc Biol 2013; 33: 2501–2508.
Robichon C, Varret M, Le Liepvre X, Lasnier F, Hajduch E, Ferre P et al. DnaJA4 is a SREBP-regulated chaperone involved in the cholesterol biosynthesis pathway. Biochim Biophys Acta 2006; 1761: 1107–1113.
Pandak WM, Hylemon PB, Ren S, Marques D, Gil G, Redford K et al. Regulation of oxysterol 7alpha-hydroxylase (CYP7B1) in primary cultures of rat hepatocytes. Hepatology 2002; 35: 1400–1408.
Quiroga AD, Li L, Trotzmuller M, Nelson R, Proctor SD, Kofeler H et al. Deficiency of carboxylesterase 1/esterase-x results in obesity, hepatic steatosis, and hyperlipidemia. Hepatology 2012; 56: 2188–2198.
Matsubara T, Kim HJ, Miyata M, Shimada M, Nagata K, Yamazoe Y . Isolation and characterization of a new major intestinal CYP3A form, CYP3A62, in the rat. J Pharmacol Exp Ther 2004; 309: 1282–1290.
Rizner TL, Penning TM . Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids 2014; 79: 49–63.
de Meijer VE, Le HD, Meisel JA, Akhavan Sharif MR, Pan A, Nose V et al. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism 2010; 59: 1092–1105.
Rees K, Dyakova M, Wilson N, Ward K, Thorogood M, Brunner E . Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev 2013; 12: CD002128.
Garshick M, Mochari-Greenberger H, Mosca L . Reduction in dietary trans fat intake is associated with decreased LDL particle number in a primary prevention population. Nutr Metab Cardiovasc Dis 2014; 24: 100–106.
Adamsson V, Cederholm T, Vessby B, Riserus U . Influence of a healthy Nordic diet on serum fatty acid composition and associations with blood lipoproteins - results from the NORDIET study. Food Nutr Res 2014; 58: 24114.
Rossmeisl M, Medrikova D, van Schothorst EM, Pavlisova J, Kuda O, Hensler M et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim Biophys Acta 2014; 1841: 267–278.
Wang J, Mitsche MA, Luetjohann D, Cohen JC, Xie XS, Hobbs HH . Relative roles of ABCG5/ABCG8 in liver and intestine. J Lipid Res 2014; 56: 319–330.
Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology 2011; 141: 1403 e1–1403 e5.
Jiang CM, Pu CW, Hou YH, Chen Z, Alanazy M, Hebbard L . Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J Gastroenterol 2014; 20: 16464–16473.
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 2011; 140: 1071–1083.
Yang X, Xu Y, Guo F, Ning Y, Zhi X, Yin L et al. Hydroxysteroid sulfotransferase SULT2B1b promotes hepatocellular carcinoma cells proliferation in vitro and in vivo. PLoS One 2013; 8: e60853.
Kim MC, Kim CS, Chung TH, Jeong J, Lee SH, Kim SR et al. MONW phenotype is associated with advanced colorectal adenoma in Korean men. Obesity (Silver Spring) 2012; 20: 1876–1881.
Acknowledgements
We thank Enzo Ceresi for technical assistance in the morphological and immunohistochemical analysis. CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of the ISCIII. This work was supported by the Spanish Government (Ministerio de Educación y Ciencia, EPIMILK (AGL2012-33692)) and by the EU FP7 project (BIOCLAIMS (FP7-244995)). Laboratory of Molecular Biology, Nutrition and Biotechnology and Human and Animal Physiology are members of the European Research Network of Excellence NuGO (The European Nutrigenomics Organization, EU Contract: FOOD-CT-2004-506360 NUGO). RD-R was recipient of a fellowship from the Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Díaz-Rúa, R., van Schothorst, E., Keijer, J. et al. Isocaloric high-fat feeding directs hepatic metabolism to handling of nutrient imbalance promoting liver fat deposition. Int J Obes 40, 1250–1259 (2016). https://doi.org/10.1038/ijo.2016.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.47
This article is cited by
-
Cognitive impairment in metabolically-obese, normal-weight rats: identification of early biomarkers in peripheral blood mononuclear cells
Molecular Neurodegeneration (2018)
-
Maternal consumption of a cafeteria diet during lactation in rats leads the offspring to a thin-outside-fat-inside phenotype
International Journal of Obesity (2017)