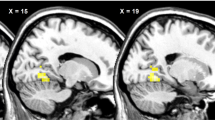
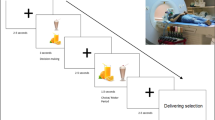
Abstract
It remains unknown whether obese individuals with more components of the metabolic syndrome and/or prediabetes demonstrate altered activation of brain centers in response to food cues. We examined obese individuals with prediabetes (n=26) vs obese individuals without prediabetes (n=11) using fMRI. We also performed regression analyses on the basis of the number of MetS components per subject. Obese individuals with prediabetes have decreased activation of the reward-related putamen in the fasting state and decreased activation of the salience- and reward-related insula after eating. Obese individuals with more components of MetS demonstrate decreased activation of the putamen while fasting. All these activations remain significant when corrected for BMI, waist circumference (WC), HbA1c and gender. Decreased activation in the reward-related central nervous system areas among the obese is more pronounced in subjects with prediabetes and MetS. Prospective studies are needed to quantify their contributions to the development of prediabetes/MetS and to study whether they may predispose to the exacerbation of obesity and the development of comorbidities over time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ko BJ, Park KH, Mantzoros CS . Diet patterns, adipokines, and metabolism: where are we and what is next? Metabolism 2014; 63: 168–177.
Mathew H, Farr OM, Mantzoros CS . Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism 2016; 65: 73–80.
Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL . Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr 2014; 1: 7.
Farr OM, Li CS, Mantzoros CS . Central nervous system regulation of eating: Insights from human brain imaging. Metabolism 2016; 65: 699–713.
Association AD. Professional Practice Committee for the Standards of Medical Care in Diabetes-2016. Diabetes Care 2016; 39: S107–S108.
Farr OM, Fiorenza C, Papageorgiou P, Brinkoetter M, Ziemke F, Koo BB et al. Leptin therapy alters appetite and neural responses to food stimuli in brain areas of leptin-sensitive subjects without altering brain structure. J Clin Endocrinol Metab 2014; 99: E2529–E2538.
Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia 2016; 59: 954–965.
Farr OM, Tsoukas MA, Triantafyllou G, Dincer F, Filippaios A, Ko BJ et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metabolism 2016; 65: 945–953.
Farr OM, Upadhyay J, Gavrieli A, Camp M, Spyrou N, Kaye H et al. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a four week long randomized, placebo-controlled, double-blinded clinical trial. Diabetes 2016; 65: 2943–2953.
Ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Deacon CF, Holst JJ et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia 2015; 58: 2688–2698.
van Bloemendaal L, RG IJ, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014; 63: 4186–4196.
Burger KS, Stice E . Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. NeuroImage 2014; 99: 122–128.
DiLeone RJ, Taylor JR, Picciotto MR . The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 2012; 15: 1330–1335.
Wang GJ, Volkow ND, Fowler JS . The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets 2002; 6: 601–609.
Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A . Neural predictors of chocolate intake following chocolate exposure. Appetite 2015; 87: 98–107.
Stice E, Burger KS, Yokum S . Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci 2015; 35: 10316–10324.
Yoshimi K, Kumada S, Weitemier A, Jo T, Inoue M . Reward-induced phasic dopamine release in the monkey ventral striatum and putamen. PloS One 2015; 10: e0130443.
Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS et al. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care 2012; 35: 1105–1111.
Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND . Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 2008; 62: 50–61.
Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 2008; 42: 1537–1543.
Acknowledgements
These projects were supported by Harvard Clinical and Translational Science Center Grant UL1 RR025758 from the National Center for Research Resources and NIDDK DK081913. Olivia M Farr is supported by training grant 5T32HD052961. This study was in part funded by Eisai, who supported the study through an Investigator-Initiated Study grant. Eisai approved the design of the study, but had no role in study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. OMF and CSM collected the data and wrote the manuscript. OMF analyzed the data. Dr Olivia Farr is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Farr, O., Mantzoros, C. Obese individuals with more components of the metabolic syndrome and/or prediabetes demonstrate decreased activation of reward-related brain centers in response to food cues in both the fed and fasting states: a preliminary fMRI study. Int J Obes 41, 471–474 (2017). https://doi.org/10.1038/ijo.2016.231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.231