Abstract
Objective:
Although intrauterine nutritional stress is known to result in offspring obesity and the metabolic phenotype, the underlying cellular/molecular mechanisms remain incompletely understood. We tested the hypothesis that compared with the controls, the bone marrow-derived mesenchymal stem cells (BMSCs) of the intrauterine growth-restricted (IUGR) offspring exhibit a more adipogenic phenotype.
Methods:
A well-established rat model of maternal food restriction (MFR), that is, 50% global caloric restriction during the later-half of pregnancy and ad libitum diet following birth that is known to result in an obese offspring with a metabolic phenotype was used. BMSCs at 3 weeks of age were isolated, and then molecularly and functionally profiled.
Results:
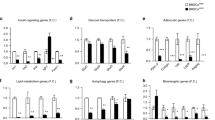
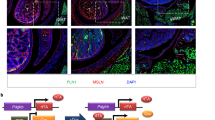
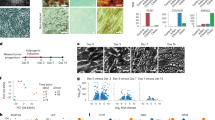
BMSCs of the intrauterine nutritionally-restricted offspring demonstrated an increased proliferation and an enhanced adipogenic molecular profile at miRNA, mRNA and protein levels, with an overall up-regulated PPARγ (miR-30d, miR-103, PPARγ, C/EPBα, ADRP, LPL, SREBP1), but down-regulated Wnt (LRP5, LEF-1, β-catenin, ZNF521 and RUNX2) signaling profile. Following adipogenic induction, compared with the control BMSCs, the already up-regulated adipogenic profile of the MFR BMSCs, showed a further increased adipogenic response.
Conclusions:
Markedly enhanced adipogenic molecular profile and increased cell proliferation of MFR BMSCs suggest a possible novel cellular/mechanistic link between the intrauterine nutritional stress and offspring metabolic phenotype. This provides new potential predictive and therapeutic targets against these conditions in the IUGR offspring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Garver WS, Newman SB, Gonzales-Pacheco DM, Castillo JJ, Jelinek D, Heidenreich RA et al. The genetics of childhood obesity and interaction with dietary macronutrients. Genes Nutr 2013; 8: 271–287.
Harding JE . The nutritional basis of the fetal origins of adult disease. Int J Epidemiol 2001; 30: 15–23.
Barker DJ, Osmond C . Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 1: 1077–1081.
Hales CN, Barker DJ . Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35: 595–601.
Tarantal AF, Berglund L . Obesity and lifespan health—importance of the fetal environment. Nutrients 2014; 6: 1725–1736.
Martin RJ, Hausman GJ, Hausman DB . Regulation of adipose cell development in utero. Proc Soc Exp Biol Med 1998; 219: 200–210.
Nombela-Arrieta C, Ritz J, Silberstein LE . The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 2011; 12: 126–131.
Lowe CE, O'Rahilly S, Rochford JJ . Adipogenesis at a glance. J Cell Sci 2011; 124: 2681–2686.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147.
Tontonoz P, Hu E, Spiegelman BM . Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev 1995; 5: 571–576.
Paek DS, Sakurai R, Saraswat A, Li Y, Khorram O, Torday JS et al. Metyrapone alleviates deleterious effects of maternal food restriction on lung development and growth of rat offspring. Reprod Sci 2015; 22: 207–222.
Rehan VK, Li Y, Corral J, Saraswat A, Husain S, Dhar A et al. Metyrapone blocks maternal food restriction-induced changes in female rat offspring lung development. Reprod Sci 2014; 21: 517–525.
Desai M, Gayle D, Babu J, Ross MG . Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol 2005; 288: R91–R96.
Desai M, Gayle D, Babu J, Ross MG . The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol 2007; 196: 555–557.
Karadag A, Sakurai R, Wang Y, Guo P, Desai M, Ross MG et al. Effect of maternal food restriction on fetal rat lung lipid differentiation program. Pediatr Pulmonol 2009; 44: 635–644.
Tseng FW, Tsai MJ, Yu LY, Fu YS, Huang WC, Cheng H . Comparative effects of bone marrow mesenchymal stem cells on lipopolysaccharide-induced microglial activation. Oxid Med Cell Longev 2013; 2013: 234179.
Miranda SC, Silva GA, Hell RC, Martins MD, Alves JB, Goes AM . Three-dimensional culture of rat BMMSCs in a porous chitosan-gelatin scaffold: A promising association for bone tissue engineering in oral reconstruction. Arch Oral Biol 2011; 56: 1–15.
Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med 2007; 175: 1158–1164.
Morales E, Sakurai R, Husain S, Paek D, Gong M, Ibe B et al. Nebulized PPARgamma agonists: a novel approach to augment neonatal lung maturation and injury repair in rats. Pediatr Res 2014; 75: 631–640.
Sharbati-Tehrani S, Kutz-Lohroff B, Bergbauer R, Scholven J, Einspanier R . miR-Q: a novel quantitative RT-PCR approach for the expression profiling of small RNA molecules such as miRNAs in a complex sample. BMC. Mol Biol 2008; 9: 34.
Bilkovski R, Schulte DM, Oberhauser F, Gomolka M, Udelhoven M, Hettich MM et al. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J Biol Chem 2010; 285: 6170–6178.
Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A . Adipogenesis and WNT signalling. Trends Endocrinol Metab 2009; 20: 16–24.
Laudes M . Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 2011; 46: R65–R72.
Addison WN, Fu MM, Yang HX, Lin Z, Nagano K, Gori F et al. Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol Cell Biol 2014; 34: 3076–3085.
Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N et al. A twist code determines the onset of osteoblast differentiation. Dev Cell 2004; 6: 423–435.
Muruganandan S, Roman AA, Sinal CJ . Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 2009; 66: 236–253.
Kobayashi H, Gao Y, Ueta C, Yamaguchi A, Komori T . Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro. Biochem Biophys Res Commun 2000; 273: 630–636.
Kang T, Lu W, Xu W, Anderson L, Bacanamwo M, Thompson W et al. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem 2013; 288: 34394–34402.
Gnanalingham MG, Mostyn A, Symonds ME, Stephenson T . Ontogeny and nutritional programming of adiposity in sheep: potential role of glucocorticoid action and uncoupling protein-2. Am J Physiol Regul Integr Comp Physiol 2005; 289: R1407–R1415.
Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 2009; 18: 4046–4053.
Desai M, Guang H, Ferelli M, Kallichanda N, Lane RH . Programmed upregulation of adipogenic transcription factors in intrauterine growth-restricted offspring. Reprod Sci 2008; 15: 785–796.
Joss-Moore LA, Wang Y, Campbell MS, Moore B, Yu X, Callaway CW et al. Uteroplacental insufficiency increases visceral adiposity and visceral adipose PPARgamma2 expression in male rat offspring prior to the onset of obesity. Early Hum Dev 2010; 86: 179–185.
Slawik M, Vidal-Puig AJ . Adipose tissue expandability and the metabolic syndrome. Genes Nutr 2007; 2: 41–45.
Bluher M . Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab 2013; 27: 163–177.
Oreffo RO, Lashbrooke B, Roach HI, Clarke NM, Cooper C . Maternal protein deficiency affects mesenchymal stem cell activity in the developing offspring. Bone 2003; 33: 100–107.
Wu CL, Diekman BO, Jain D, Guilak F . Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int J Obes (Lond) 2013; 37: 1079–1087.
Nobili V, Alisi A, Panera N, Agostoni C . Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev 2008; 6: 241–247.
Acknowledgements
This research was funded by grants from the NIH (HD058948, HL107118, HD071731, HD127237) and the TRDRP (17RT-0170, and 23RT-0018). The authors had full access to all of the data and take responsibility for the integrity of the data and the accuracy of analysis.
Author contributions
VKR conceived this work. MG, SA, RS, and JL carried out the experiments. VKR, MG, RS, MI analyzed the data. VKR, MG and SA drafted the paper. All authors have the approval of the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gong, M., Antony, S., Sakurai, R. et al. Bone marrow mesenchymal stem cells of the intrauterine growth-restricted rat offspring exhibit enhanced adipogenic phenotype. Int J Obes 40, 1768–1775 (2016). https://doi.org/10.1038/ijo.2016.157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.157
This article is cited by
-
ZNF521 Has an Inhibitory Effect on the Adipogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells
Stem Cell Reviews and Reports (2018)