Abstract
Background/Objectives:
Brown adipose tissue (BAT) is a potential therapeutic target against obesity and diabetes through thermogenesis and substrate disposal with cold exposure. The role of BAT in energy metabolism under thermoneutral conditions, however, remains controversial. We assessed the contribution of BAT to energy expenditure (EE), particularly diet-induced thermogenesis (DIT), and substrate utilization in human adults.
Methods:
In this cross-sectional study, BAT activity was evaluated in 21 men using 18F-fluoro-2-deoxy-D-glucose positron emission tomography combined with computed tomography (18F-FDG-PET/CT) after cold exposure (19 °C). The subjects were divided into BAT-positive (n=13) and BAT-negative (n=8) groups according to the 18F-FDG-PET/CT findings. Twenty-four hour EE, DIT and respiratory quotient were measured using a whole-room indirect calorimeter at 27 °C.
Results:
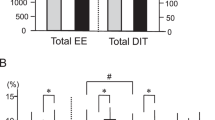
Body composition, blood metabolites and 24-h EE did not differ between groups. DIT (%), calculated as DIT divided by total energy intake, however, was significantly higher in the BAT-positive group (BAT-positive: 9.7±2.5%, BAT-negative: 6.5±4.0%, P=0.03). The 24-h respiratory quotient was significantly lower (P=0.03) in the BAT-positive group (0.861±0.027) than in the BAT-negative group (0.889±0.024).
Conclusion:
DIT and fat utilization were higher in BAT-positive subjects compared to BAT-negative subjects, suggesting that BAT has a physiologic role in energy metabolism.
Similar content being viewed by others
Introduction
Brown adipose tissue (BAT) is an important regulatory tissue for core body temperature and whole-body energy expenditure (EE) in small rodents and human infants.1 Recent human studies using 18F-fluoro-2-deoxy-D-glucose-positron emission tomography in combination with computed tomography (18F-FDG-PET/CT) revealed that adult humans also have active BAT.2, 3, 4, 5 Based on several cross-sectional studies, human BAT decreases with age and is inversely correlated with body mass index or visceral fat area.2, 3, 6, 7, 8 The age-dependent decrease in BAT might be associated with an accumulation of body weight and fat.6, 8
Active BAT has a protective role against obesity and diabetes through the regulation of cold-induced thermogenesis (CIT).9, 10 Yoneshiro et al.9 reported that CIT, evaluated based on the difference between resting EE at a thermoneutral temperature (27 °C) and EE at a non-shivering cold temperature (19 °C), increases by up to 12% in subjects with metabolically active BAT. Chondronikola et al.10 also reported that prolonged cold exposure (5–8 h) with a liquid cooling vest (20 °C) increases resting EE, whole-body glucose disposal and insulin sensitivity in those with active BAT. These findings suggest that BAT in adult humans has a role in whole-body EE and substrate metabolism, at least under cold conditions. Such non-shivering cold exposure, however, is uncommon under free-living conditions in more temperate climates. Further investigation is required to clarify the contribution of BAT to whole-body EE and substrate utilization under thermoneutral and free-living conditions.
BAT contains uncoupling protein 1 (UCP1), which mediates the uncoupling of mitochondrial substrate oxidation from ATP production, leading to the release of energy as heat from free fatty acid oxidation.1 Activation of BAT is dependent on UCP1 expression and the sympathetic nervous system (SNS) through β3-adrenoreceptors.11 The SNS is stimulated by cold, diet, stress and inflammation, which leads to increases in obligatory and/or facultative thermogenesis and may explain how BAT contributes to adaptive thermogenesis, including diet-induced thermogenesis (DIT) and CIT.11, 12 The role of BAT in DIT in adult humans is not clear, but a polymorphism in UCP1 (-3826A/G) is associated with lower postprandial EE.13 Moreover, UCP1 gene polymorphisms also accelerate age-related decreases in BAT activity.14 Thus, BAT may have a specific role in postprandial thermogenesis to produce heat from fatty acid oxidation, but this has not yet been demonstrated directly.15, 16 More recently, Peterson et al.17 examined the thermic response to a 50% overfeeding diet following a 4-week cold intervention. Despite increases in CIT and SNS activity, DIT was unchanged in response to 50% overfeeding. On the other hand, Lee et al.18 demonstrated that BAT recruitment is accompanied by enhanced DIT and postprandial insulin sensitivity. Thus, the role of BAT in energy metabolism in free-living humans remains controversial.
In the present study, to clarify the relationship among BAT activity, EE and substrate utilization in humans, we compared 24-h EE, DIT and whole-body substrate oxidation under free-living conditions between subjects with and without metabolically active BAT using a whole-room indirect calorimeter. We hypothesized that BAT-positive subjects would have higher DIT and substrate utilization than BAT-negative subjects.
Subjects and methods
Subjects
Twenty-one healthy men were recruited through poster advertisements and word of mouth. Inclusion criteria were as follows: 20–50 years of age and a body mass index of 18.0–24.9 (kgm−2). Participants were excluded if they had a history of, or required, medication for cardiovascular disease, hypertension, diabetes, hypercholesterolemia, hyperglycemia, hyperlipidemia, eating disorders, smoking, excessive alcohol intake (>30 g alcohol/d), allergies to ingredients in the test food, or worked as a shift worker or experienced a weight change of more than 2.0 kg during the 2 months prior to the trial. The Institutional Review Boards of the National Institute of Health and Nutrition, Tenshi College, National Center for Global Health and Medicine, and Kao Corporation approved the study protocol. All subjects provided written informed consent before study commencement.
18F-FDG-PET/CT
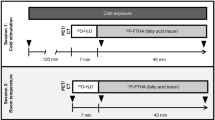
During the winter (between late December and early March) from 2013 to 2015 in Japan, BAT activity in the subjects was measured using 18F-FDG-PET/CT. Subjects were assessed in the morning after fasting for 6–12 h. The subjects wore light clothes (T-shirt and shorts) and remained in a room adjusted to 19 °C for 2 h while intermittently placing a towel-wrapped ice block against the soles of their feet. After 1 h, 18F-FDG (1.66–5.18 MBq per kg body weight) was administered intravenously and the subjects remained in the same cold conditions for another hour.2, 6, 8 One hour after the administration of 18F-FDG, a whole-body PET/CT scan was performed at 24 °C using a dedicated PET/CT system (either Aquiduo (Toshiba Medical Systems, Otawara, Japan), Biograph 16 (Siemens Medical Solutions, Knoxville, TN, USA), or Discovery PET/CT 600 (GE Healthcare, Waukesha, WI, USA)). Low-dose CT without contrast enhancement was performed first and used for attenuation correction and anatomic image fusion. The PET data were reconstructed using a Gaussian filter with an ordered-subset expectation maximization algorithm. The iteration and subset parameters were determined based on the manufacturer’s recommendations. A quality control study was performed to minimize the standardized uptake value (SUV) difference between the three PET/CT systems using a standard National Electrical Manufacturers Association body phantom. FDG uptake by BAT lesions was semi-quantitatively measured as the maximum SUV (SUVmax), defined as the tissue radioactivity concentration divided by the injection dose per body weight, using a workstation adapted for each PET/CT system (either VOX-BASE (J-MAC System, Sapporo, Japan), e-Soft (Siemens) or Xeleris (GE Healthcare)). The SUVmax threshold level between the BAT-positive and BAT-negative groups was set at 2.00.4, 6
Anthropometrics and body composition analysis
Body weight was measured using an Ohaus digital scale accurate to 0.01 kg (CQ100LW, Ohaus Corp., Parsippany, NJ, USA) after the subjects exited the calorimeter. Body composition was then measured by whole-body dual-energy X-ray absorptiometry (QRD 4500 W, Hologic Inc., Waltham, MA, USA). Absolute fat mass (FM), fat-free mass (FFM) and body fat percentage were determined with Hologic Systems Software using the procedures outlined in the Hologic QRD 4500 User’s Guide. The body fat percentage was calculated according to the following formula: BF%=FM/(FM+FFM) × 100.
Measurements of energy metabolism
The experiment was a cross-sectional study for a series of measurements in winter (between late December and early March) from 2013 to 2015, within 3 weeks after BAT measurement by FDG-PET/CT, and 24-h energy metabolism was measured using the whole-room indirect calorimeter. Subjects arrived at Kao Corporation, a chemical, cosmetic and food company headquartered in Japan (Tokyo, Japan), by 1800 hours and ate the prescribed meal. At 1900 hours, the subjects entered the calorimeter and energy metabolism was measured for 24 h from 0000 hours. Subjects ate the same meal in exactly the same volume at 0900 hours (breakfast), 1400 hours (lunch) and 1900 hours (dinner), and were allowed to drink water freely. The meal ingredients were weighed and cooked by a registered dietitian in the metabolic kitchen. Energy intake (EI) was individually adjusted from the basal metabolic rate (BMR) predicted using the formula by Ganpule et al.19 based on subject age, sex, height and body weight, and multiplied by 1.3 as the limited physical activity level. The three meals were identical and comprised a rice bowl with grilled seafood and pork, tofu, and miso-soup without any spice, containing 15 energy percent protein, 25 energy percent fat and 60 energy percent carbohydrate (CHO). The energy composition and amount of each of the three meals was exactly the same. Subjects went to bed at 0000 hours and got up at 0700 hours. The time from 0715 to 0900 hours was used for resting and a low-intensity activity program (e.g., personal computer work, jigsaw puzzles and folding laundry were performed for 20 min, respectively). For the remaining time, the subjects were allowed free activity so as to maintain a situation similar to free-living. Sleeping other than at the scheduled time was not allowed.
Whole-room indirect calorimeter
Twenty-four hour calorimetric measurements were performed using a whole-room indirect calorimeter (Fuji Medical Science Co. Ltd., Chiba, Japan).20 Air in the chamber was pumped out at a rate of 100 L per min. Temperature and relative humidity of the incoming fresh air were maintained at 27.0±0.2 °C and 50.0±3.0%, respectively. The air samples were dried using a gas-sampling unit (CPF-8000, Shimadzu Corp., Kyoto, Japan) and analyzed using a mass spectrometer (VG PRIMA δB, Thermo Fisher Scientific, Cheshire, UK), which was calibrated biweekly using standard gas. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were calculated using the formula of Henning et al.21 The VO2 and VCO2 data were offset by 2 min to adjust for the response lag time of the respiratory measurements. VO2 and VCO2 were calculated over 30 min to obtain the EE and respiratory quotient (RQ). Based on the 24-h VO2 and VCO2, as well as nitrogen excretion, total EE (TEE) and RQ were calculated.22, 23 Physical activity levels were monitored using an infrared motion sensor (AMP2009B01, Matsushita Automation Controls Co. Ltd., Tokyo, Japan), which measured the percentage of time during each minute that movement was detected. The sleeping metabolic rate (SMR) was calculated as the lowest mean EE over a continuous 3-h sleeping period. BMR was calculated using the formula: BMR=SMR/0.94.19 Energy balance was calculated by subtracting TEE from EI. Activity-induced thermogenesis (AIT) was calculated for 0700–0859 hours and 0900–2359 hours, and DIT for 0900–2359 hours was calculated by plotting EE against radar output averaged over 30-min periods according to the method of Schutz et al.24 The intercept of the regression line at the lowest radar output of the infrared motion sensor corresponds to EE during inactivity, that is, resting metabolic rate, which comprised BMR and DIT. The difference between the resting metabolic rate and BMR represents the DIT of meals when computed over 15 h. The DIT (%) was defined as postprandial thermogenesis divided by food intake, and calculated as DIT divided by the total EI.25
Dietary record and food quotient (FQ)
Subjects were instructed by a registered dietitian to record their food intake. To record habitual food intake, subjects recorded the contents of the meals consumed during the 3 days prior to the calorimeter stay. FQ was measured for the 3 days before measurement of the energy metabolism. The nutritional value of the meals was calculated by the dietitian based on the fifth revised edition of the Standard Tables of Food Composition in Japan. The FQ was calculated using the following formula:26
FQ={0.207* × CHO (%)+0.159* × fat (%)+0.193* × protein (%)}/
{0.207* × CHO (%)+0.226* × fat (%)+0.243* × protein (%)}
Blood and urine metabolites
Fasting blood samples for insulin, serum glucose, thyroid-stimulating hormone, thyroid hormone (T3, T4), triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, free fatty acid, leptin, adiponectin, interleukin-6 and glucagon-like protein-1, were collected from each subject and centrifuged at 1500 × g for 15 min at 4 °C after the subjects exited the whole-room calorimeter at 0830 hours. All serum samples were stored at −80 °C until analysis. All measurements were performed by LSI Medience Corporation (Tokyo, Japan). During the measurement in the whole-room indirect calorimeter, all urine was collected in the following periods: Period 1: 0000–0700 hours, Period 2: 0701–1359 hours, Period 3: 1400–1859 hours, and Period 4: 1900–2359 hours. The urine was weighed, and urinary norepinephrine, epinephrine and dopamine were hydrolyzed. The concentration of each was calculated as the total of the free and conjugated forms using an automated catecholamine analyzer (HLC-725CAII, Tosoh, Tokyo, Japan), multiplied by the urine volume as the total elimination.
Statistical analysis
Data are presented as mean±s.d. unless otherwise indicated. The mean body composition, EI and blood metabolite values were evaluated, and the difference between the two groups was compared by two-tailed Student’s t-test. According to the power calculations, for the primary outcome measure of DIT, nine subjects in each study group were required for a power of 80% with a two-sided alpha of 0.05 to detect a BAT-positive and BAT-negative difference of 74 (kcal per 15 h) with a standard deviation of 51 based on our preliminary study. Considering drop-out and study group imbalance, a total of 22 subjects was required. Because 24-h energy balance during the whole-room calorimeter stay and FQ during the 3 days prior to the calorimeter measurements are reported to have a strong effect on RQ,26 24-h RQ was adjusted for these factors. RQ was assessed using analysis of variance (ANOVA) adjusted for FQ and energy balance as covariates. The changes in EE per FFM (EE/FFM) and RQ were plotted as accumulated values per 30 min, and analyzed using repeated measures ANOVA. The correlation between RQ and BAT activity was analyzed using Pearson’s correlation. Urinary catecholamines were recorded as the total elimination for each time period, and analyzed using repeated measures ANOVA. P-values of less than 0.05 were considered statistically significant. SAS software for Windows (Ver. 9.2, SAS Institute, Cary, NC, USA) was used for the statistical analyses.
Results
Subject characteristics
Based on the 18FDG uptake in the supraclavicular region and quantitation by calculating SUVmax (threshold of 2.00), 13 subjects (62%) were classified as BAT-positive and 8 subjects as BAT-negative. Mean SUVmax was 8.5±4.8 in the BAT-positive group and 1.1±0.4 in the BAT-negative group (P<0.001). The data of the 13 BAT-negative subjects and 8 BAT-positive subjects were analyzed. Although the number of subjects in the study groups was imbalanced, the statistical power was 86.6%, which is sufficient for an explanatory study. Table 1 shows the subjects’ body composition and blood metabolite data. The two groups did not differ significantly in age, body mass index, FFM, FM, or glucose, lipid and hormone levels. All subjects had a body mass index within the range of 19.1–24.6 kgm−2, and none of the subjects was obese. None of the subjects had abnormal blood glucose, cholesterol or triglyceride levels. In the 3 days before the whole-room calorimeter measurement, total EI (BAT-negative: 2354±406 kcal d−1 vs BAT-positive: 2115±363 kcal d−1, P=0.18), protein intake (79.8±18.1 vs 70.7±13.8 g d−1, P=0.21), fat intake (78.5±12.7 vs 72.5±24.7 g d−1, P=0.53), CHO intake (309.3±59.3 vs 276.3±41.3 g d−1, P=0.15) and FQ (0.871±0.013 vs 0.872±0.021, P=0.96) were not significantly difference between groups.
EE and substrate utilization
Table 2 shows the 24-h EE and its components in the whole-room indirect calorimeter at room temperature (27 °C). The mean SMR of all subjects was 1437±107 (kcal/d), and SMR was strongly and positively correlated with individual FFM (r=0.803, P<0.001). Mean DIT (%) of all subjects was 6.5±3.3%, which was very close to the values measured by whole-room indirect calorimetry and calculated in other studies27, 28 using the same method. TEE and energy balance did not differ significantly between groups. Similarly, there was no difference in the SMR, activity-induced thermogenesis, TEE/FFM ratio or physical activity levels. DIT for 15 h tended to be higher in the BAT-positive group (P=0.06). DIT (%) was significantly higher in the BAT-positive group than in the BAT-negative group (P=0.03; Figure 1). Figure 2a shows the change in EE/FFM accumulated over 30-min intervals. The ANOVA revealed that EE/FFM had a significant group × time interaction (P=0.010) and time effect (P<0.001), but no main effect (P=0.339). The EE/FFM during 1100–1130 hours and 2200–2230 hours was greater in the BAT-positive group than in the BAT-negative group.
Changes in (a) energy expenditure (EE) per fat free mass (FFM) and (b) RQ during 30-min intervals in a whole-room indirect calorimeter for 24 h. Data are expressed as value per hour±s.e. Black circles with black lines represent the BAT-positive group (n=13) and the white squares with black lines represent the BAT-negative group (n=8). Grey triangles represent the meal times (0900, 1400 and 1900 hours). (A) ANOVA showed that EE/FFM over 24 h varied with group and time as demonstrated by the lack of a significant effect of group (P=0.339), but a significant effect of time (P<0.001) and a group × time interaction (P=0.010). (b) ANOVA showed that RQ over 24 h varied with group and time as demonstrated by the significant effect of group (P=0.029) and the significant effect of time (P<0.001), but no significant group × time interaction (P=0.010). * significantly different from BAT-negative group (P<0.05, without corrections for multiple comparisons).
The 24-h RQ was significantly lower (P=0.03) in the BAT-positive group than in the BAT-negative group (Table 2). The RQ adjusted for the covariates 24-h energy balance and FQ for the 3 days prior to the measurements, was significantly lower (P=0.04) in the BAT-positive group (0.861±0.027) than in the BAT-negative group (0.889±0.024). When examined over 30-min intervals throughout the 24-h period, a significant main effect of the BAT condition was observed for RQ (P=0.03), which was significantly decreased in the BAT-positive subjects compared with BAT-negative subjects at 0030–0130 hours, 0530–0630 hours, 0900–1100 hours, 1300–1430 hours, 1600–1700 hours, 1830–2030 hours and 22:0–2400 hours (Figure 2b). A significant effect of time was detected (P<0.001), but the group × time interaction was not significant (P=0.161).
We also analyzed the relationship between BAT activity and energy metabolism in all subjects. Correlation analysis conducted between energy metabolism and BAT activity (SUVmax) revealed a significant negative correlation between the 24-h RQ and SUVmax (Figure 3). No significant correlation was detected between DIT (%), TEE and SUVmax (r=0.34, P=0.141, n=21 and r=−0.09, P=0.678, n=21, respectively).
Urinary catecholamines
Table 3 shows the urinary norepinephrine, epinephrine and dopamine levels. Norepinephrine, epinephrine and dopamine excretion did not differ significantly between groups and there was no group × time interaction. Twenty-four hour urinary norepinephrine, epinephrine and dopamine levels did not differ significantly between groups.
Discussion
In the present study, we evaluated the contribution of BAT to EE, particularly DIT and substrate utilization, in human daily life. Measurements of TEE, activity-induced thermogenesis, DIT, SMR and RQ were obtained in 21 healthy BAT-positive and BAT-negative subjects using a whole-room indirect calorimeter. Our findings revealed that (1) BAT-positive subjects had a significantly higher DIT (%) compared with BAT-negative subjects; and (2) BAT-positive subjects had a lower RQ compared with BAT-negative subjects. These findings indicate that metabolically active BAT contributes at least partially to DIT under thermoneutral conditions, and increases energy use from fatty acids. These results support the idea of BAT as a potential target for treating obesity and diabetes, even under free-living and thermoneutral conditions.
The present findings are the first to demonstrate a DIT increase of 56 kcal/d in BAT-positive subjects, and that DIT (%), expressed as DIT divided by total EI, differed significantly between BAT-positive and BAT-negative subjects under thermoneutral conditions (9.7 and 6.5%, respectively). DIT is the EE necessary to digest, absorb, transfer and store food nutrients, and is generally determined by the food components and total EI. Despite many studies, the mechanism of the association of DIT with obesity and other diseases remain unknown.29 Vosselman et al.15 evaluated BAT activity after a high-carbohydrate meal using 18FDG-PET/CT and DIT by indirect calorimetry. In young non-obese males, they observed that FDG uptake is higher after a high-fat meal compared to a fasting state, but did not observe a direct relationship between BAT activity and DIT. Few human studies have attempted to measure the contribution of BAT to DIT using whole-room indirect calorimetry and 18F-FDG-PET/CT.17, 18 Lee et al.18 measured DIT following 4-weeks cold exposure, and found that BAT recruitment is accompanied by an increase in DIT. Peterson et al.17 demonstrated in nine healthy men that cold acclimation results in increased CIT, but not a higher DIT response to 150% overfeeding. These inconsistent results could be explained by methodologic differences and/or relatively small sample sizes. The study by Peterson et al.17 was a longitudinal single-arm trial and did not directly measure BAT activity. The study by Lee et al.18 reported high values of DIT in excess of 30%, which might include mixed values of DIT and CIT after four weeks of intervention. In the present study, the contribution of BAT to DIT was compared between BAT-positive and BAT-negative individuals using whole-room indirect calorimetry. This is basically the same concept as the experiments comparing UCP1-deficient mice with normal mice with regard to the role of BAT in small animals.30, 31 Our observations using this method in the present study indicate that active BAT contributes to postprandial thermogenesis, even under free-living and thermoneutral conditions, and suggest that BAT is a potential target for treating obesity.
Although EE/FFM changes over 24 h revealed no main effects between groups, the EE/FFM changes showed a significant BAT-time interaction, with a significant increase after breakfast and dinner (Figure 2a). In addition, although not statistically significant, EE/FFM was higher overnight among BAT-positive subjects, especially just before rising. Lee et al. recently demonstrated that BAT exhibits a biorhythm in humans.32 These findings together suggest that the EE/FFM differences between BAT-positive and BAT-negative subjects should be interpreted in light of a possible circadian rhythm. The accumulated EE data adjusted for individual FFM for 24 h was not different between groups. Moreover, BAT activity was not significantly correlated with DIT, suggesting that the contribution of BAT to TEE and daily energy balance is limited.
Studies in rodents indicate increased mitochondrial uncoupling in BAT after food intake33 and increased oxygen consumption.34 The phenomenon is controlled by the SNS, and the norepinephrine concentration is increased after eating meals.35, 36, 37 Although we did not investigate the blood norepinephrine concentration after eating, there was no difference in the daily urinary norepinephrine excretion between the BAT-positive and BAT-negative groups. These findings suggest that the SNS response to food intake was similar in the two groups. Therefore, the higher DIT in the BAT-positive group was more likely to be associated with BAT activity than with SNS activity. Our observations seem consistent with findings that people with higher BAT activity have higher CIT, but similar SNS responses, compared to those with lower BAT activities.38, 39
Our study also revealed that the 24-h RQ was significantly lower in the BAT-positive group than in the BAT-negative group, suggesting that fat utilization was higher in BAT-positive subjects than in BAT-negative subjects. Moreover, the change in RQ was significantly different between BAT-negative and BAT-positive subjects, with a significant decrease around meal times (Figure 2b). After adjusting for the 24-h energy balance and FQ before the calorimetry measurement, the RQ was lower in BAT-positive subjects, suggesting higher fat utilization in BAT. Pasanisi et al. reported that BAT activity was inversely correlated with RQ in seven constitutionally lean young women.40 They suggested that their findings support the hypothesis that BAT has a protective role against fat mass accumulation, mostly because of the preferential use of lipids as a substrate in resting thermoneutral conditions. An experiment in mice revealed that BAT mainly utilizes fat as energy under sympathetic nerve stimulation.41 Our findings indicate that in humans, too, individuals with higher BAT activity have higher fat utilization, even at room temperature. This is an important finding, and suggests functional activity of BAT in human adults under thermoneutral conditions. Furthermore, Zurlo et al.42 reported that the 24-h RQ predicts subsequent changes in body weight based on findings of the 24-h RQ using a whole-room indirect calorimeter in non-diabetic Pima Indians. Eckel et al.43 and Ellis et al.44 reported similar results. These findings indicated that a higher RQ correlates with future weight gain. Pannacciulli et al.45 also reported that a higher RQ and a high amount of CHO oxidation increase body weight over a subsequent 3-day ad libitum period through an increase in caloric intake.45 This may partially explain the association between the presence/absence of BAT and the prevalence of obesity in middle-aged and older populations.6, 7 In other words, individuals in whom fat is not utilized by BAT are more likely to gain weight and/or have impaired insulin sensitivity.
A limitation of this study is a potential residual confounding effect due to an interaction between BAT activity and health status in healthy Japanese male subjects. Although we attempted to account for these unknown confounding effects by adjusting for several metabolites, dietary record and physical activity, we found no correlations between these factors. In addition, the urinary catecholamines as a measure of the SNS response to food intake may not be precise.
In conclusion, the findings of the present study, namely that DIT and fat utilization are higher in BAT-positive individuals, suggest a functional role of BAT in daily life, and may also explain how obesity progresses in BAT-negative individuals.
References
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011; 19: 1755–1760.
Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18 F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011; 96: 192–199.
Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M . Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2014; 38: 812–817.
Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011; 19: 13–16.
Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014; 63: 4089–4099.
Chernogubova E, Hutchinson DS, Nedergaard J, Bengtsson T . Alpha1- and beta1-adrenoceptor signaling fully compensates for beta3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology 2005; 146: 2271–2284.
van Marken Lichtenbelt WD, Schrauwen P . Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol 2011; 301: R285–R296.
Nagai N, Sakane N, Ueno LM, Hamada T, Moritani T . The −3826A—>G variant of the uncoupling protein-1 gene diminishes postprandial thermogenesis after a high fat meal in healthy boys. J Clin Endocrinol Metab 2003; 88: 5661–5667.
Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T et al. Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int J Obes (Lond) 2013; 37: 993–998.
Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM et al. Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr 2013; 98: 57–64.
Schlogl M, Piaggi P, Thiyyagura P, Reiman EM, Chen K, Lutrin C et al. Overfeeding over 24 hours does not activate brown adipose tissue in humans. J Clin Endocrinol Metab 2013; 98: E1956–E1960.
Peterson CM, Lecoultre V, Frost EA, Simmons J, Redman LM, Ravussin E . The thermogenic responses to overfeeding and cold are differentially regulated. Obesity (Silver Spring) 2016; 24: 96–101.
Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 2014; 63: 3686–3698.
Ganpule AA, Tanaka S, Ishikawa-Takata K, Tabata I . Interindividual variability in sleeping metabolic rate in Japanese subjects. Eur J Clin Nutr 2007; 61: 1256–1261.
Hibi M, Masumoto A, Naito Y, Kiuchi K, Yoshimoto Y, Matsumoto M et al. Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am J Physiol Regul Integr Comp Physiol 2013; 304: R94–R101.
Henning B, Lofgren R, Sjostrom L . Chamber for indirect calorimetry with improved transient response. Med Biol Eng Comput 1996; 34: 207–212.
Brouwer E . On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl 1957; 6: 795–802.
Weir JB . New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9.
Schutz Y, Bessard T, Jequier E . Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr 1984; 40: 542–552.
Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J . Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab 2013; 98: 2791–2799.
Toubro S, Sorensen TI, Hindsberger C, Christensen NJ, Astrup A . Twenty-four-hour respiratory quotient: the role of diet and familial resemblance. J Clin Endocrinol Metab 1998; 83: 2758–2764.
Usui C, Ando T, Ohkawara K, Miyake R, Oshima Y, Hibi M et al. Validity and reproducibility of a novel method for time-course evaluation of diet-induced thermogenesis in a respiratory chamber. Physiol Rep 2015; 3: e12410.
Tataranni PA, Larson DE, Snitker S, Ravussin E . Thermic effect of food in humans: methods and results from use of a respiratory chamber. Am J Clin Nutr 1995; 61: 1013–1019.
Granata GP, Brandon LJ . The thermic effect of food and obesity: discrepant results and methodological variations. Nutr Rev 2002; 60: 223–233.
Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997; 387: 90–94.
Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M . Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 2005; 54: 1385–1391.
Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A et al. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab 2016; 12: 602–609.
Lupien JR, Glick Z, Saito M, Bray GA . Guanosine diphosphate binding to brown adipose tissue mitochondria is increased after single meal. Am J Physiol 1985; 249: R694–R698.
Glick Z, Teague RJ, Bray GA . Brown adipose tissue: thermic response increased by a single low protein, high carbohydrate meal. Science 1981; 213: 1125–1127.
Glick Z, Raum WJ . Norepinephrine turnover in brown adipose tissue is stimulated by a single meal. Am J Physiol 1986; 251: R13–R17.
Schwartz RS, Jaeger LF, Silberstein S, Veith RC . Sympathetic nervous system activity and the thermic effect of feeding in man. Int J Obesity 1987; 11: 141–149.
Welle S, LIlavivat U, Campbell RG . Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism 1981; 30: 953–958.
Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011; 14: 272–279.
van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013; 123: 3395–3403.
Pasanisi F, Pace L, Fonti R, Marra M, Sgambati D, De Caprio C et al. Evidence of brown fat activity in constitutional leanness. J Clin Endocrinol Metab 2013; 98: 1214–1218.
Fedorenko A, Lishko PV, Kirichok Y . Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012; 151: 400–413.
Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990; 259: E650–E657.
Eckel RH, Hernandez TL, Bell ML, Weil KM, Shepard TY, Grunwald GK et al. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr 2006; 83: 803–808.
Ellis AC, Hyatt TC, Hunter GR, Gower BA . Respiratory quotient predicts fat mass gain in premenopausal women. Obesity (Silver Spring) 2010; 18: 2255–2259.
Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J . The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 2007; 86: 625–632.
Acknowledgements
We thank Ms Hiroko Yamaguchi and Ms Tomomi Yamazaki for their excellent work as the dietary staff of Kao Health Care Food Research Laboratories. We also thank Mr Satoshi Nakae and Dr Takafumi Ando of National Institute of Health and Nutrition, for the data collection. We thank Mr Hironobu Miyauchi and Mr Shigeru Nakajima of Fuji Medical Science Co. Ltd., for their technical expertise in respiratory chamber measurements. Sources of support: This work was financed by Grants-in-Aid for Scientific Research (A) (Grant Number 24240092 to S.T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Clinical Trial Registry number and website: This trial was registered at www.umin.ac.jp as UMIN000019364.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Masanobu Hibi, Sachiko Oishi, Tohru Yamaguchi, Koichi Yasunaga and Yoshihisa Katsuragi are employees of the Kao Corporation. The other authors have no personal or financial conflicts of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hibi, M., Oishi, S., Matsushita, M. et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes 40, 1655–1661 (2016). https://doi.org/10.1038/ijo.2016.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.124
This article is cited by
-
An overview of the complex interaction between obesity and target organ dysfunction: focus on redox-inflammatory state
Nutrire (2023)
-
Diurnal variations of brown fat thermogenesis and fat oxidation in humans
International Journal of Obesity (2021)
-
The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue
Nature Communications (2021)
-
Activation of brown adipose tissue in diet-induced thermogenesis is GC-C dependent
Pflügers Archiv - European Journal of Physiology (2020)
-
Activation of brown adipose tissue enhances the efficacy of caloric restriction for treatment of nonalcoholic steatohepatitis
Laboratory Investigation (2019)