Abstract
Background/Objectives:
Consumption of high-energy beverages has been implicated as a risk factor for weight gain, yet why nutrients ingested as beverages fail to generate adequate satiety remains unclear. In general, consumers do not expect drinks to be satiating, but drinks generate greater satiety when their sensory characteristics imply they may be filling. These findings challenge traditional bottom-up models of how gut-based satiety signals modify behaviour to suggest that beliefs at the point of ingestion modify gut-based satiety signalling.
Subjects/Methods:
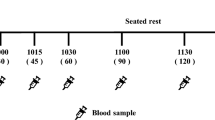
Healthy volunteers (n=23) consumed four different beverages, combining an overt sensory manipulation (thin, low sensory (LS) or thicker and more creamy, enhanced sensory (ES)) and covert nutrient manipulation (low energy (LE), 78 kcal; high energy (HE), 267 kcal) on different days. Effects on satiety were assessed through rated appetite and levels of glucose, insulin, pancreatic polypeptide (PP) and cholesystokinin (CCK) recorded periodically over 90 min, and through intake at an ad libitum test lunch.
Results:
Intake at the test lunch and rated appetite were both altered by both the sensory and nutrient manipulations, with lowest intake and greatest suppression of hunger post-drink in the ESHE condition. Insulin increased more after HE than LE drinks, and after ES than LS drinks, whereas PP levels were higher after ES than LS versions. CCK levels only increased after the ESHE drink.
Conclusions:
These data confirm acute sensitivity of satiety after consuming a drink both to the sensory characteristics and nutrient content of the drink, and suggest that this may be, at least in part, due to top-down modulation of release of satiety-related gut hormones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hill JO, Wyatt HR, Peters JC . Energy balance and obesity. Circulation 2012; 126: 126–132.
Sclafani A, Ackroff K . Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol 2012; 302: R1119–R1133.
Sam AH, Troke RC, Tan TM, Bewick GA . The role of the gut/brain axis in modulating food intake. Neuropharmacology 2012; 63: 46–56.
Hussain S, Bloom S . The regulation of food intake by the gut–brain axis: implications for obesity. Int J Obes 2013; 37: 625–633.
Hellström PM . Satiety signals and obesity. Curr Opin Gastroenterol 2013; 29: 222–227.
Perry B, Wang Y . Appetite regulation and weight control: the role of gut hormones. Nutr Diabetes 2012; 2: e26.
Holzer P, Reichmann F, Farzi A, . Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut–brain axis. Neuropeptides 2012; 46: 261–274.
Stubbs J, Whybrow S Beverages, appetite and energy balance. In: Wilson T Temple NJ (eds). Beverages in Nutrition and Health. Humana Press: Totowa, NJ, USA, 2003, pp 261–278.
de Graaf C . Why liquid energy results in overconsumption. P Nutr Soc 2011; 70: 162–170.
Mattes R . Soup and satiety. Physiol Behav 2005; 83: 739–747.
Pavlov IP, Gantt WH, Volborth G, Cannon WB . Conditioned Reflexes and Psychiatry, vol 2. International Publishers: New York, NY, USA, 1941.
Woods SC . The eating paradox: how we tolerate food. Psychol Rev 1991; 98: 488–505.
Teff KL, Mattes RD, Engelman K . Cephalic phase insulin release in normal weight males: verification and reliability. Am J Physiol 1991; 261: E430–E436.
Teff KL, Mattes RD, Engelman K, Mattern J . Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism 1993; 42: 1600–1608.
Berthoud H, Jeanrenaud B . Sham feeding-induced cephalic phase insulin release in the rat. Am J Physiol 1982; 242: E280–E285.
Bernstein IL, Woods SC . Ontogeny of cephalic insulin release by the rat. Physiol Behav 1980; 24: 529–532.
Teff KL, Devine J, Engelman K . Sweet taste: effect on cephalic phase insulin release in men. Physiol Behav 1995; 57: 1089–1095.
Just T, Pau HW, Engel U, Hummel T . Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 2008; 51: 622–627.
Teff KL . Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans. Physiol Behav 2010; 99: 317–323.
Teff K . Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite 2000; 34: 206–213.
Schwartz T, Stenquist B, Olbe L . Cephalic phase of pancreatic-polypeptide secretion studied by sham feeding in man. Scand J Gastroenterol 1979; 14: 313–320.
Smeets PAM, Erkner A, de Graaf C . Cephalic phase responses and appetite. Nutr Rev 2010; 68: 643–655.
Schwartz T, Holst J, Fahrenkrug J, Jensen SL, Nielsen OV, Rehfeld J et al. Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest 1978; 61: 781.
Schwartz TW . Pancreatic polypeptide: a unique model for vagal control of endocrine systems. J Auton Nerv Syst 1983; 9: 99–111.
Taylor I, Feldman M . Effect of cephalic–vagal stimulation on insulin, gastric inhibitory polypeptide, and pancreatic polypeptide release in humans*. J Clin Endocrinol Metab 1982; 55: 1114–1117.
Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology 2003; 124: 1325–1336.
Kojima S, Ueno N, Asakawa A, Sagiyama K, Naruo T, Mizuno S et al. A role for pancreatic polypeptide in feeding and body weight regulation. Peptides 2007; 28: 459–463.
Jayasena CN, Bloom SR . Role of gut hormones in obesity. Endocrin Metab Clin 2008; 37: 769–787.
Cassady BA, Considine RV, Mattes RD . Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr 2012; 95: 587–593.
Chambers L, McCrickerd K, Yeomans MR . Optimising foods for satiety. Trends Food Sci Technol 2015; 41: 149–160.
Chambers L, Ells H, Yeomans MR . Can the satiating power of a high energy beverage be improved by manipulating sensory characteristics and label information? Food Qual Prefer 2013; 28: 271–278.
Yeomans MR, Chambers LC . Satiety-relevant sensory qualities enhance the satiating effects of mixed carbohydrate-protein preloads. Am J Clin Nutr 2011; 94: 1410–1417.
Yeomans MR, McCrickerd K, Brunstrom JM, Chambers L . Effects of repeated consumption on sensory-enhanced satiety. Brit J Nutr 2014; 111: 1137–1144.
McCrickerd K, Chambers L, Yeomans MR . Fluid or fuel? The context of consuming a beverage is important for satiety. PLoS One 2014; 9: e100406.
Stunkard AJ, Messick S . The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83.
Hull S, Re R, Tiihonen K, Viscione L, Wickham M . Consuming polydextrose in a mid-morning snack increases acute satiety measurements and reduces subsequent energy intake at lunch in healthy human subjects. Appetite 2012; 59: 706–712.
Hull S, Re R, Chambers L, Echaniz A, Wickham MS . A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur J Nutr 2014; 1–8.
Blundell J, De Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev 2010; 11: 251–270.
McCrickerd K, Chambers L, Brunstrom JM, Yeomans MR . Subtle changes in the flavour and texture of a drink enhance expectations of satiety. Flavour 2012; 1: 20.
McCrickerd K, Chambers L, Yeomans MR . Does modifying the thick texture and creamy flavour of a drink change portion size selection and intake? Appetite 2014; 73: 114–120.
Morgan L, Tredger J, Wright J, Marks V . The effect of soluble- and insoluble-fibre supplementation on post-prandial glucose tolerance, insulin and gastric inhibitory polypeptide secretion in healthy subjects. Br J Nutr 1990; 64: 103–110.
Jenkins DJ, Axelsen M, Kendall CW, Augustin LS, Vuksan V, Smith U . Dietary fibre, lente carbohydrates and the insulin-resistant diseases. Br J Nutr 2000; 83: S157–S163.
Slavin J, Green H . Dietary fibre and satiety. Nutr Bull 2007; 32: S32–S42.
Batterham R, Le Roux C, Cohen M, Park A, Ellis S, Patterson M et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 2003; 88: 3989–3992.
Berntson GG, Zipf WB, O'Dorisio TM, Hoffman JA, Chance RE . Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides 1993; 14: 497–503.
Adrian T, Bloom S, Bryant M, Polak J, Heitz P, Barnes A . Distribution and release of human pancreatic polypeptide. Gut 1976; 17: 940–944.
Little T, Horowitz M, Feinle‐Bisset C . Role of cholecystokinin in appetite control and body weight regulation. Obes Rev 2005; 6: 297–306.
Murphy KG, Bloom SR . Gut hormones and the regulation of energy homeostasis. Nature 2006; 444: 854–859.
Chaudhri OB, Salem V, Murphy KG, Bloom SR . Gastrointestinal satiety signals. Annu Rev Physiol 2008; 70: 239–255.
Crum AJ, Corbin WR, Brownell KD, Salovey P, Mind Over . Milkshakes: mindsets, not just nutrients, determine ghrelin response. Health Psychol 2011; 30: 424–429.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This study was conducted as part of research grant BB/H004645/1 from the UK Biotechnology and Biological Sciences Research Council (BBSRC) as part of the DRINC initiative. The authors declare no conflict of interest relating to the outcomes of the reported study.
Rights and permissions
About this article
Cite this article
Yeomans, M., Re, R., Wickham, M. et al. Beyond expectations: the physiological basis of sensory enhancement of satiety. Int J Obes 40, 1693–1698 (2016). https://doi.org/10.1038/ijo.2016.112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.112
This article is cited by
-
The importance of the olfactory system in human well-being, through nutrition and social behavior
Cell and Tissue Research (2021)
-
Food texture influences on satiety: systematic review and meta-analysis
Scientific Reports (2020)