Abstract
Background:
Osteoblast-specific secreted osteocalcin has been considered as an important regulator of energy and glucose metabolism, however, the causative role and clinical potential of osteocalcin implicated in insulin resistance remains not fully understood.
Methods:
Osteocalcin was administered intermittently in vivo and in vitro, and metabolic parameters, autophagy and insulin signaling were assessed.
Results:
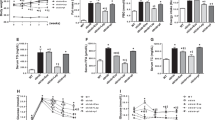
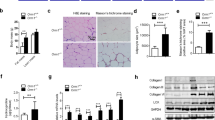
The intermittent injections of osteocalcin in mice fed high-fat diet resulted in decreased body weight gain, fat-pad weight gain, serum triglycerides, serum-free fatty acid, blood glucose, insulin level and partial normalization of glucose tolerance relative to the mice fed high-fat diet and received vehicle injections. Meanwhile, the intermittent administration of osteocalcin not only led to the alleviation of autophagic dysfunction and endoplasmic reticulum (ER) stress, but also contributed to the restoration of the impaired insulin signaling in adipose tissue and skeleton muscle of mice consumed the high-fat diet. In accordance with these findings in vivo, osteocalcin treatment also displayed a protective impact on adipocytes and myocytes against tunicamycin- or palmitate-induced ER stress and autophagy dysfunction in an XBP-1-independent manner, with these effects of osteocalcin being reversed by inhibition of mammalian target of rapamycin (mTOR) or nuclear factor-κB (NF-κB).
Conclusions:
Intermittent administration of osteocalcin efficiently reversed the attenuated autophagy and ER stress, and restored the impaired insulin sensitivity in cellular and mice models of insulin resistance. Our findings provide new insights into the clinical potential of osteocalcin in metabolic homeostasis, and suggest an innovative strategy for the treatment against diabetes, obesity and metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Epstein S . Serum and urinary markers of bone remodelling: assessment of bone turnover. Endocrine Rev 1988; 9: 437–449.
Fukumoto S, Martin TJ . Bone as an endocrine organ. Trends Endocrinol Metab 2009; 20: 230–236.
de Paula FJ, Horowitz MC, Rosen CJ . Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev 2010; 26: 622–630.
Razzaque MS . Osteocalcin: a pivotal mediator or an innocent bystander in energy metabolism? Nephrol Dial Transplant 2011; 26: 42–45.
Ferron M, Hinoi E, Karsenty G, Ducy P . Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 2008; 105: 5266–5270.
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G . Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012; 50: 568–575.
Hauschka PV, Lian JB, Cole DE, Gundberg CM . Osteocalcin and matrix Gla protein:vitamin K-dependent proteins in bone. Physiol Rev 1989; 69: 990–1047.
Price PA . Gla-containing proteins of bone. Connect Tissue Res 1989; 21: 51–57.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007; 130: 456–469.
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 2007; 94: 45–49.
Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol 2009; 161: 723–729.
Diamanti-Kandarakis E, Livadas S, Katsikis I, Piperi C, Mantziou A, Papavassiliou AG et al. Serum concentrations of carboxylated osteocalcin are increased and associated with several components of the polycystic ovarian syndrome. J Bone Miner Metab 2010; 29: 201–206.
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 2011; 22: 187–194.
Kim SH, Lee JW, Im JA, Hwang HJ . Serum osteocalcin is related to abdominal obesity in Korean obese and overweight men. Clin Chim Acta 2010; 411: 2054–2057.
Bulló M, Moreno-Navarrete JM, Fernández-Real JM, Salas-Salvadó J . Total and undercarboxylated osteocalcin predict changes in insulin sensitivity and β cell function in elderly men at high cardiovascular risk. Am J Clin Nutr 2012; 95: 249–255.
Winhofer Y, Handisurya A, Tura A, Bittighofer C, Klein K, Schneider B et al. Osteocalcin is related to enhanced insulin secretion in gestational diabetes mellitus. Diabetes Care 2010; 33: 139–143.
Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP et al. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol 2010; 163: 265–272.
Yang L, Li P, Fu S, Calay ES, Hotamisligil GS . Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 2010; 11: 467–478.
Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controlls osteogenic differentiation of human mesenchymal stem cells. Bone 2013; 52: 524–531.
Jang WG, Kim EJ, Koh JT . Tunicamycin negatively regulates BMP2-induced osteoblast differentiation through CREBH expression in MC3T3E1 cells. BMB Rep 2011; 44: 735–740.
Zhou B, Li H, Xu L, Zang W, Wu S, Sun H . Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-κB signaling pathway. Endocrinology 2013; 154: 1055–1068.
Sun HZ, Yang TW, Zang WJ, Wu SF . Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. J Endocrinol 2010; 204: 311–318.
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109–1122.
Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on Huntingtin-induced cell death. J Cell Biol 2005; 171: 603–614.
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 2004; 21: 81–93.
Wu S, Morrison A, Sun H, De Luca F . Nuclear factor-kappaB (NF-kappaB) p65 interacts with Stat5b in growth plate chondrocytes and mediates the effects of growth hormone on chondrogenesis and on the expression of insulin-like growth factor-1 and bone morphogenetic protein-2. J Biol Chem 2011; 286: 24726–24734.
Li H, Zhou B, Xu L, Liu J, Zang W, Wu S et al. Circulating PGRN is significantly associated with systemic insulin sensitivity and autophagic activity in metabolicsyndrome. Endocrinology 2014; 155: 3493–3507.
Liu J, Li H, Zhou B, Xu L, Kang X, Yang W et al. PGRN induces impaired insulin sensitivity and defective autophagy in hepatic insulin resistance. Mol Endocrinol 2015; 29: 528–541.
Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med 2013; 19: 83–92.
Mondal AK, Das SK, Varma V, Nolen GT, McGehee RE, Elbein SC et al. Effect of endoplasmic reticulum stress on inflammation and adiponectin regulation in human adipocytes. Metab Syndr Relat Disord 2012; 10: 297–306.
Kovsan J, Blüher M, Tarnovscki T, Klöting N, Kirshtein B, Madar L et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab 2011; 96: E268–E277.
Zhou L, Zhang J, Fang Q, Liu M, Liu X, Jia W et al. Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Mol Pharmacol 2009; 76: 596–603.
Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S . Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role inadipogenesis. Proc Natl Acad Sci USA 2009; 24: 19860–19865.
Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE et al. Endocrine regulation of male fertility by the skeleton. Cell 2011; 144: 796–809.
Yang S, Xu H, Yu S, Cao H, Fan J, Ge C et al. Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J Biol Chem 2011; 286: 19149–19158.
Zhang W, Shen X, Wan C, Zhao Q, Zhang L, Zhou Q et al. Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: differential signaling via Akt and ERK. Cell Biochem Funct 2012; 30: 297–302.
Wang W, Zhang X, Zheng J, Yang J . High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol Cell Biochem 2010; 338: 115–122.
Zhao S, Zhu L, Duan H, Liu S, Liu Q, Liu W et al. PI3K/Akt pathway mediates high glucose-induced lipid accumulation in human renal proximal tubular cells via spliced XBP-1. J Cell Biochem 2012; 113: 3288–3298.
Zhou B, Li H, Liu J, Xu L, Zang W, Wu S et al. Intermittent injections of osteocalcin reverses autophagic dysfunction and endoplasmic reticulum stress resulting from diet-induced obesity in the vascular tissue via the NF-κB-p65-dependent mechanism. Cell Cycle 2013; 12: 1901–1903.
Acknowledgements
We appreciate the technical support from the electron microscope center of Xi’an Jiaotong University. This work was supported by the programs from the National Natural Science Foundation of China (general program no. 30930105, no. 30971392, no. 81071440, no. 81170741, no. 81370899, no. 81472038 and no. 81500016), National Excellent Young Scientist Program (no. 81222026) and the New Century Excellent Talents in University from the Ministry of Education, China (NCET-08-0435).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhou, B., Li, H., Liu, J. et al. Autophagic dysfunction is improved by intermittent administration of osteocalcin in obese mice. Int J Obes 40, 833–843 (2016). https://doi.org/10.1038/ijo.2016.1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.1
This article is cited by
-
Bupivacaine Induces ROS-Dependent Autophagic Damage in DRG Neurons via TUG1/mTOR in a High-Glucose Environment
Neurotoxicity Research (2022)
-
Acute continuous moderate-intensity exercise, but not low-volume high-intensity interval exercise, attenuates postprandial suppression of circulating osteocalcin in young overweight and obese adults
Osteoporosis International (2019)
-
Uncarboxylated Osteocalcin Enhances Glucose Uptake Ex Vivo in Insulin-Stimulated Mouse Oxidative But Not Glycolytic Muscle
Calcified Tissue International (2018)
-
RETRACTED ARTICLE: Progranulin causes adipose insulin resistance via increased autophagy resulting from activated oxidative stress and endoplasmic reticulum stress
Lipids in Health and Disease (2017)