Abstract
Background/Objectives:
Mounting evidence supports a link between circadian disruption and metabolic disease. Humans with circadian disruption (for example, night-shift workers) have an increased risk of obesity and cardiometabolic diseases compared with the non-disrupted population. However, it is unclear whether the obesity and obesity-related disorders associated with circadian disruption respond to therapeutic treatments as well as individuals with other types of obesity.
Subjects/Methods:
Here, we test the effectiveness of the commonly used bariatric surgical procedure, Vertical Sleeve Gastrectomy (VSG), in mouse models of genetic and environmental circadian disruption.
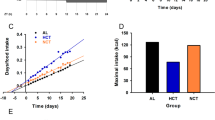
Results:
VSG led to a reduction in body weight and fat mass in both ClockΔ19 mutant and constant-light mouse models (P<0.05), resulting in an overall metabolic improvement independent of circadian disruption. Interestingly, the decrease in body weight occurred without altering diurnal feeding or activity patterns (P>0.05). Within circadian-disrupted models, VSG also led to improved glucose tolerance and lipid handling (P<0.05).
Conclusions:
Together these data demonstrate that VSG is an effective treatment for the obesity associated with circadian disruption, and that the potent effects of bariatric surgery are orthogonal to circadian biology. However, as the effects of bariatric surgery are independent of circadian disruption, VSG cannot be considered a cure for circadian disruption. These data have important implications for circadian-disrupted obese patients. Moreover, these results reveal new information about the metabolic pathways governing the effects of bariatric surgery as well as of circadian disruption.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ogden CL, Carroll MD, Kit BK, Flegal KM . Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief 2012; 82: 1–8.
Sandoval D . Bariatric surgeries: beyond restriction and malabsorption. Int J Obes (Lond) 2011; 35: S45–S49.
Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 2011; 141: 950–958.
Bessler M, Daud A, Kim T, DiGiorgi M . Prospective randomized trial of banded versus nonbanded gastric bypass for the super obese: early results. Surg Obes Relat Dis 2007; 3: 480–484.
Knutsson A . Health disorders of shift workers. Occup Med (Lond) 2003; 53: 103–108.
Ketchum ES, Morton JM . Disappointing weight loss among shift workers after laparoscopic gastric bypass surgery. Obes Surg 2007; 17: 581–584.
Bass J, Takahashi JS . Circadian integration of metabolism and energetics. Science 2010; 330: 1349–1354.
Ma Y, Bertone ER, Stanek EJ 3rd, Reed GW, Hebert JR, Cohen NL et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 2003; 158: 85–92.
Berkey CS, Rockett HR, Gillman MW, Field AE, Colditz GA . Longitudinal study of skipping breakfast and weight change in adolescents. Int J Obes Relat Metab Disord 2003; 27: 1258–1266.
O'Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res 2004; 12: 1789–1796.
Scheer FA, Hilton MF, Mantzoros CS, Shea SA . Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009; 106: 4453–4458.
Ohta H, Yamazaki S, McMahon DG . Constant light desynchronizes mammalian clock neurons. Nat Neurosci 2005; 8: 267–269.
Pittendrigh CS, Daan S . A functional analysis of circadian pacemakers in nocturnal rodents: V. Pacemaker structure: A clock for all seasons. J Comp Physiol A 1976; 106: 333–355.
Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J 2013; 27: 1721–1732.
Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK et al. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav 2011; 105: 120–123.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188.
Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 2010; 138 : 2426–2436 2436.e1–3.
Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 2013; 144 : e5.
Rand CS, Macgregor AM, Stunkard AJ . The night eating syndrome in the general population and among postoperative obesity surgery patients. Int J Eat Disord 1997; 22: 65–69.
Allison KC, Wadden TA, Sarwer DB, Fabricatore AN, Crerand CE, Gibbons LM et al. Night eating syndrome and binge eating disorder among persons seeking bariatric surgery: prevalence and related features. Obesity (Silver Spring) 2006; 14: 77S–82S.
Colles SL, Dixon JB . Night eating syndrome: impact on bariatric surgery. Obes Surg 2006; 16: 811–820.
Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, Westerterp-Plantenga MS . Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr 2012; 96: 689–697.
Cain SW, Filtness AJ, Phillips CL, Anderson C . Enhanced preference for high-fat foods following a simulated night shift. Scand J Work Environ Health 2015; 41: 288–293.
de Assis MA, Kupek E, Nahas MV, Bellisle F . Food intake and circadian rhythms in shift workers with a high workload. Appetite 2003; 40: 175–183.
Arble DM, Sandoval DA, Seeley RJ . Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia 2015; 58: 211–220.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 2009; 106: 2365–2370.
Mukherji A, Kobiita A, Ye T, Chambon P . Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013; 153: 812–827.
Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH et al. Circadian disorganization alters intestinal microbiota. PLoS One 2014; 9: e97500.
Acknowledgements
Grant Support: This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (F32 DK097867-01, D.Arble; DK082480-01, D.Sandoval; and R01 DK093848-01, R.Seeley).
Author Contributions
DMA designed and performed experiments, analyzed data and wrote the manuscript. DAS, FWT, SCW and RJS contributed to experimental design and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DMA, FWT and SCW have no conflicts to declare. DAS receives funding from Ethicon Endo-Surgery, Novo Nordisk and Boehringer Ingelheim International. RJS receives funding from Ethicon Endo-Surgery, Ablaris Therapeutics, Inc., Novo Nordisk, Novartis, Angiochem, Eisai, Forest Pharmaceuticals, Givaudan, Zealand Pharmaceuticals and Boehringer Ingelheim International.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Arble, D., Sandoval, D., Turek, F. et al. Metabolic effects of bariatric surgery in mouse models of circadian disruption. Int J Obes 39, 1310–1318 (2015). https://doi.org/10.1038/ijo.2015.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.54
This article is cited by
-
Do bariatric patient’s in rural areas achieve comparative weight loss as national average? single center experience in appalachia west virginia
Surgical Endoscopy (2022)
-
Intravital imaging of islet Ca2+ dynamics reveals enhanced β cell connectivity after bariatric surgery in mice
Nature Communications (2021)