Abstract
Background:
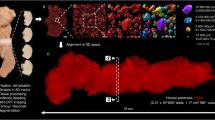
Obesity and insulin resistance lead to islet hyperplasia. However, how the islet remodeling influences the pancreatic environment and the associated neurovascular networks is largely unknown. The lack of information is primarily due to the difficulty of global visualization of the hyperplasic islet (>200 μm) and the neurovascular environment with high definition.
Methods:
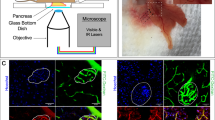
We modulated the pancreatic optical property to achieve 3-dimensional (3-D) whole-islet histology and to integrate transmitted light microscopy (which provides the ground-truth tissue information) with confocal fluorescence imaging. The new optical and imaging conditions were used to globally examine the hyperplastic islets of the young (2 months) obese db/db and ob/ob mice, which otherwise cannot be easily portrayed by the standard microtome-based histology. The voxel-based islet micrographs were digitally processed for stereo projection and qualitative and quantitative analyses of the islet tissue networks.
Results:
Paired staining and imaging of the pancreatic islets, ducts and neurovascular networks reveal the unexpected formation of the ‘neuro-insular-ductal complex’ in the young obese mice. The complex consists of the peri- and/or intra-islet ducts and prominent peri-ductal sympathetic nerves; the latter contributes to a marked increase in islet sympathetic innervation. In vascular characterization, we identify a decreased perivascular density of the ob/ob islet pericytes, which adapt to ensheathing the dilated microvessels with hypertrophic processes.
Conclusions:
Modulation of pancreatic optical property enables 3-D panoramic examination of islets in the young hyperphagic mice to reveal the formation of the islet–duct complex and neurovascular remodeling. On the basis of the morphological proximity of the remodeled tissue networks, we propose a reactive islet microenvironment consisting of the endocrine cells, ductal epithelium and neurovascular tissues in response to the metabolic challenge that is experienced early in life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
D'Adamo E, Caprio S . Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 2011; 34: S161–S165.
Chen L, Magliano DJ, Zimmet PZ . The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 2012; 8: 228–236.
Linnemann AK, Baan M, Davis DB . Pancreatic beta-cell proliferation in obesity. Adv Nutr 2014; 5: 278–288.
Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D . Role of the forkhead protein FoxO1 in beta cell compensation to insulin resistance. J Clin Invest 2006; 116: 775–782.
Woods SC, Porte D Jr . Neural control of the endocrine pancreas. Physiol Rev 1974; 54: 596–619.
Ahren B . Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 2000; 43: 393–410.
Ahren B . Islet nerves in focus—defining their neurobiological and clinical role. Diabetologia 2012; 55: 3152–3154.
Richards OC, Raines SM, Attie AD . The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev 2010; 31: 343–363.
Hayden MR, Karuparthi PR, Habibi J, Lastra G, Patel K, Wasekar C et al. Ultrastructure of islet microcirculation, pericytes and the islet exocrine interface in the HIP rat model of diabetes. Exp Biol Med (Maywood) 2008; 233: 1109–1123.
Bergers G, Song S . The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005; 7: 452–464.
Ahren B, Holst JJ . The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 2001; 50: 1030–1038.
Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962.
Creager MA, Luscher TF, Cosentino F, Beckman JA . Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003; 108: 1527–1532.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412.
Giugliano D, Ceriello A, Paolisso G . Oxidative stress and diabetic vascular complications. Diabetes Care 1996; 19: 257–267.
Vincent AM, Russell JW, Low P, Feldman EL . Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 2004; 25: 612–628.
Teitelman G, Guz Y, Ivkovic S, Ehrlich M . Islet injury induces neurotrophin expression in pancreatic cells and reactive gliosis of peri-islet Schwann cells. J Neurobiol 1998; 34: 304–318.
Chiu YC, Hua TE, Fu YY, Pasricha PJ, Tang SC . 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia 2012; 55: 3252–3261.
Tang SC, Chiu YC, Hsu CT, Peng SJ, Fu YY . Plasticity of Schwann cells and pericytes in response to islet injury in mice. Diabetologia 2013; 56: 2424–2434.
Juang JH, Kuo CH, Peng SJ, Tang SC . 3-D imaging reveals participation of donor islet Schwann cells and pericytes in islet transplantation and graft neurovascular regeneration. Ebiomedicine 2015; 2: 109–119.
Dai C, Brissova M, Reinert RB, Nyman L, Liu EH, Thompson C et al. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes 2013; 62: 4144–4153.
Slack JM . Developmental biology of the pancreas. Development 1995; 121: 1569–1580.
Dor Y, Brown J, Martinez OI, Melton DA . Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004; 429: 41–46.
Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC . Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia 2010; 53: 21–26.
Fu YY, Lin CW, Enikolopov G, Sibley E, Chiang AS, Tang SC . Microtome-free 3-dimensional confocal imaging method for visualization of mouse intestine with subcellular-level resolution. Gastroenterology 2009; 137: 453–465.
Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ et al. Structural and molecular interrogation of intact biological systems. Nature 2013; 497: 332–337.
Marx V . Microscopy: seeing through tissue. Nat Methods 2014; 11: 1209–1214.
Tang SC, Peng SJ, Chien HJ . Imaging of the islet neural network. Diabetes Obes Metab 2014; 16 (Supp 1): 77–86.
Willyard C . Heritability: the family roots of obesity. Nature 2014; 508: S58–S60.
Fernandez JR, Klimentidis YC, Dulin-Keita A, Casazza K . Genetic influences in childhood obesity: recent progress and recommendations for experimental designs. Int J Obes (Lond) 2012; 36: 479–484.
Hinney A, Vogel CI, Hebebrand J . From monogenic to polygenic obesity: recent advances. Eur Child Adolesc Psychiatry 2010; 19: 297–310.
Kanasaki K, Koya D . Biology of obesity: lessons from animal models of obesity. J Biomed Biotechnol 2011; 2011: 197636.
Llewellyn CH, Trzaskowski M, van Jaarsveld CH, Plomin R, Wardle J . Satiety mechanisms in genetic risk of obesity. JAMA Pediatr 2014; 168: 338–344.
Fu YY, Lu CH, Lin CW, Juang JH, Enikolopov G, Sibley E et al. Three-dimensional optical method for integrated visualization of mouse islet microstructure and vascular network with subcellular-level resolution. J Biomed Opt 2010; 15: 046018.
Fu YY, Peng SJ, Lin HY, Pasricha PJ, Tang SC . 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol 2013; 304: G1–11.
Liu YA, Chung YC, Shen MY, Pan ST, Kuo CW, Peng SJ et al. Perivascular interstitial cells of cajal in human colon. Cell Mol Gastroenterol Hepatol 2015; 1: 102–119.
Juang JH, Peng SJ, Kuo CH, Tang SC . Three-dimensional islet graft histology: panoramic imaging of neural plasticity in sympathetic reinnervation of transplanted islets under the kidney capsule. Am J Physiol Endocrinol Metab 2014; 306: E559–E570.
Genina EA, Bashkatov AN, Tuchin VV . Tissue optical immersion clearing. Expert Rev Med Devices 2010; 7: 825–842.
Liu YA, Chung YC, Pan ST, Hou YC, Peng SJ, Pasricha PJ et al. 3-D illustration of network orientations of interstitial cells of Cajal subgroups in human colon as revealed by deep-tissue imaging with optical clearing. Am J Physiol Gastrointest Liver Physiol 2012; 302: G1099–G1110.
Hua TE, Yang TL, Yang WC, Liu KJ, Tang SC . 3-D neurohistology of transparent tongue in health and injury with optical clearing. Front Neuroanat 2013; 7: 36.
Toma H, Winston J, Micci MA, Shenoy M, Pasricha PJ . Nerve growth factor expression is up-regulated in the rat model of L-arginine-induced acute pancreatitis. Gastroenterology 2000; 119: 1373–1381.
Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes 2004; 53: 3107–3114.
Cooke JP, Oka RK . Does leptin cause vascular disease? Circulation 2002; 106: 1904–1905.
Sikka G, Yang R, Reid S, Benjo A, Koitabashi N, Camara A et al. Leptin is essential in maintaining normal vascular compliance independent of body weight. Int J Obes (Lond) 2010; 34: 203–206.
Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV et al. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes 2006; 55: 3335–3343.
Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI et al. Mouse models of diabetic neuropathy. Neurobiol Dis 2007; 28: 276–285.
Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC . Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol 2008; 295: H1634–H1641.
Sharma K, McCue P, Dunn SR . Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol 2003; 284: F1138–F1144.
Tulachan SS, El-Gohary Y, Gittes GK . Pancreatic duct-to-β-cell transdifferentiation represents the most likely source of new beta cells during postnatal growth and regeneration. Gastroenterology 2014; 146 (Suppl 1): S81–S82.
Means AL, Ray KC, Singh AB, Washington MK, Whitehead RH, Harris RC Jr . et al. Overexpression of heparin-binding EGF-like growth factor in mouse pancreas results in fibrosis and epithelial metaplasia. Gastroenterology 2003; 124: 1020–1036.
Krakowski ML, Kritzik MR, Jones EM, Krahl T, Lee J, Arnush M et al. Pancreatic expression of keratinocyte growth factor leads to differentiation of islet hepatocytes and proliferation of duct cells. Am J Pathol 1999; 154: 683–691.
Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 2007; 133: 1999–2009.
Xiao X, Prasadan K, Guo P, El-Gohary Y, Fischbach S, Wiersch J et al. Pancreatic duct cells as a source of VEGF in mice. Diabetologia 2014; 57: 991–1000.
Borden P, Houtz J, Leach SD, Kuruvilla R . Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep 2013; 4: 287–301.
Talchai C, Lin HV, Kitamura T, Accili D . Genetic and biochemical pathways of beta-cell failure in type 2 diabetes. Diabetes Obes Metab 2009; 11: 38–45.
Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001; 153: 543–553.
Acknowledgements
We thank the Brain Research Center in National Tsing Hua University for technical support of confocal imaging. This work was supported in part by grants from the Chang Gung Memorial Hospital (CMRP G3C0191 and CMRPG3D0601) to JHJ, and Taiwan National Health Research Institutes (NHRI-EX102-10044EI and NHRI-EX103-10332EI) and National Science Council (NSC 102-2628-B-007-002-MY2) to SCT.
Author contributions
All authors contributed to experimental design, data analysis and interpretation of data. HJC, SJP and TEH contributed to specimen preparation and data collection. CHK and JHJ contributed to the islet transplantation experiment. SCT directed the imaging project and contributed to the writing of the paper. All the authors approved the final version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Chien, HJ., Peng, SJ., Hua, TE. et al. 3-D imaging of islets in obesity: formation of the islet–duct complex and neurovascular remodeling in young hyperphagic mice. Int J Obes 40, 685–697 (2016). https://doi.org/10.1038/ijo.2015.224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.224
This article is cited by
-
Local islet remodelling associated with duct lesion–islet complex in adult human pancreas
Diabetologia (2021)
-
Beta cell dysfunction in diabetes: the islet microenvironment as an unusual suspect
Diabetologia (2020)
-
Human pancreatic neuro-insular network in health and fatty infiltration
Diabetologia (2018)
-
Pancreatic neuro-insular network in young mice revealed by 3D panoramic histology
Diabetologia (2018)