Abstract
Background/Objectives:
Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) are key enzymes involved in intracellular lipid catabolism. We have previously shown decreased expression and activity of these lipases in adipose tissue of obese insulin resistant individuals. Here we hypothesized that lipase deficiency might impact on insulin sensitivity and metabolic homeostasis in adipocytes not just by enhancing lipid accumulation, but also by altering lipid and carbohydrate catabolism in a peroxisome proliferator-activated nuclear receptor (PPAR)-dependent manner.
Methods:
To address our hypothesis, we performed a series of in vitro experiments in a human white adipocyte model, the human multipotent adipose-derived stem (hMADS) cells, using genetic (siRNA) and pharmacological knockdown of ATGL and/or HSL.
Results:
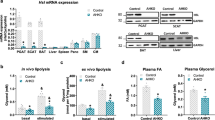
We show that ATGL and HSL knockdown in hMADS adipocytes disrupted mitochondrial respiration, which was accompanied by a decreased oxidative phosphorylation (OxPhos) protein content. This lead to a reduced exogenous and endogenous palmitate oxidation following ATGL knockdown, but not in HSL deficient adipocytes. ATGL deficiency was followed by excessive triacylglycerol accumulation, and HSL deficiency further increased diacylglycerol accumulation. Both single and double lipase knockdown reduced insulin-stimulated glucose uptake, which was attributable to impaired insulin signaling. These effects were accompanied by impaired activation of the nuclear receptor PPARα, and restored on PPARα agonist treatment.
Conclusions:
The present study indicates that lipase deficiency in human white adipocytes contributes to mitochondrial dysfunction and insulin resistance, in a PPARα-dependent manner. Therefore, modulation of adipose tissue lipases may provide a promising strategy to reverse insulin resistance in obese and type 2 diabetic patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, Rodriguez A . Regulation of adipocyte lipolysis. Nutr Res Rev 2014; 27: 63–93.
Jocken JWE, Goossens GH, Blaak EE . Targeting adipose tissue lipid metabolism to improve glucose metabolism in cardiometabolic disease. EMJ Diabet 2014; 2: 73–82.
Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med 2011; 17: 1076–1085.
Mottillo EP, Bloch AE, Leff T, Granneman JG . Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) alpha and delta in brown adipocytes to match fatty acid oxidation with supply. J Biol Chem 2012; 287: 25038–25048.
Strom K, Gundersen TE, Hansson O, Lucas S, Fernandez C, Blomhoff R et al. Hormone-sensitive lipase (HSL) is also a retinyl ester hydrolase: evidence from mice lacking HSL. FASEB J 2009; 23: 2307–2316.
Zimmermann R, Haemmerle G, Wagner EM, Strauss JG, Kratky D, Zechner R . Decreased fatty acid esterification compensates for the reduced lipolytic activity in hormone-sensitive lipase-deficient white adipose tissue. J Lipid Res 2003; 44: 2089–2099.
Goossens GH . The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav 2008; 94: 206–218.
Karastergiou K, Fried SK . Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr Atheroscler Rep 2013; 15: 361.
Karpe F, Dickmann JR, Frayn KN . Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011; 60: 2441–2449.
Jocken JW, Goossens GH, van Hees AM, Frayn KN, van Baak M, Stegen J et al. Effect of beta-adrenergic stimulation on whole-body and abdominal subcutaneous adipose tissue lipolysis in lean and obese men. Diabetologia 2008; 51: 320–327.
Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 2007; 92: 2292–2299.
McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011; 60: 47–55.
Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Ryden M et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 2005; 54: 3190–3197.
Arner P, Langin D . Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab 2014; 25: 255–262.
Girousse A, Langin D . Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int J Obes (Lond) 2012; 36: 581–594.
Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J et al. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem 2009; 284: 18282–18291.
Popeijus HE, van Otterdijk SD, van der Krieken SE, Konings M, Serbonij K, Plat J et al. Fatty acid chain length and saturation influences PPARalpha transcriptional activation and repression in HepG2 cells. Mol Nutr Food Res 2014; 58: 2342–2349.
Folch J, Lees M, Sloane Stanley GH . A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497–509.
Jocken JW, Goossens GH, Boon H, Mason RR, Essers Y, Havekes B et al. Insulin-mediated suppression of lipolysis in adipose tissue and skeletal muscle of obese type 2 diabetic men and men with normal glucose tolerance. Diabetologia 2013; 56: 2255–2265.
Shepherd D, Garland PB . The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 1969; 114: 597–610.
Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 2006; 281: 40236–40241.
Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 2002; 277: 4806–4815.
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98: 115–124.
Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD . Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 2014; 99: E209–E216.
Yehuda-Shnaidman E, Buehrer B, Pi J, Kumar N, Collins S . Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes 2010; 59: 2474–2483.
Walker CG, Holness MJ, Gibbons GF, Sugden MC . Fasting-induced increases in aquaporin 7 and adipose triglyceride lipase mRNA expression in adipose tissue are attenuated by peroxisome proliferator-activated receptor alpha deficiency. Int J Obes (Lond) 2007; 31: 1165–1171.
Maeda N, Funahashi T, Hibuse T, Nagasawa A, Kishida K, Kuriyama H et al. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc Natl Acad Sci USA 2004; 101: 17801–17806.
Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel AL et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 2013; 11: e1001485.
Bakke SS, Moro C, Nikolic N, Hessvik NP, Badin PM, Lauvhaug L et al. Palmitic acid follows a different metabolic pathway than oleic acid in human skeletal muscle cells; lower lipolysis rate despite an increased level of adipose triglyceride lipase. Biochim Biophys Acta 2012; 1821: 1323–1333.
van de Weijer T, Havekes B, Bilet L, Hoeks J, Sparks L, Bosma M et al. Effects of bezafibrate treatment in a patient and a carrier with mutations in the PNPLA2 gene, causing neutral lipid storage disease with myopathy. Circ Res 2013; 112: e51–e54.
Smih F, Rouet P, Lucas S, Mairal A, Sengenes C, Lafontan M et al. Transcriptional regulation of adipocyte hormone-sensitive lipase by glucose. Diabetes 2002; 51: 293–300.
Kim JY, Tillison K, Lee JH, Rearick DA, Smas CM . The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-alpha in 3T3-L1 adipocytes and is a target for transactivation by PPARgamma. Am J Physiol Endocrinol Metab 2006; 291: E115–E127.
Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS . Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 2006; 55: 148–157.
Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M et al. Isoproterenol, TNFalpha, and insulin downregulate adipose triglyceride lipase in 3T3-L1 adipocytes. Mol Cell Endocrinol 2005; 240: 43–49.
Coppack SW, Fisher RM, Humphreys SM, Clark ML, Pointon JJ, Frayn KN . Carbohydrate metabolism in insulin resistance: glucose uptake and lactate production by adipose and forearm tissues in vivo before and after a mixed meal. Clin Sci (Lond) 1996; 90: 409–415.
Horowitz JF, Coppack SW, Klein S . Whole-body and adipose tissue glucose metabolism in response to short-term fasting in lean and obese women. Am J Clin Nutr 2001; 73: 517–522.
Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N Engl J Med 2014; 370: 2307–2315.
Acknowledgements
The authors gratefully thank all volunteers for their time and motivation, and Jos Stegen and Wendy Sluijsmans for their excellent technical support. This work was supported by a VENI grant (016.116.074) from the Netherlands Organization for Scientific Research (NWO) to JWEJ and a clinical research grant from the European Foundation for the Study of Diabetes (EFSD) to EEB and JWEJ.
Author contributions
All clinical studies were performed and samples were obtained in the laboratory of EEB (Maastricht, the Netherlands) and analytical experiments were conducted at the laboratories of EEB. (Maastricht, the Netherlands). Substantial contribution to concept and design, acquisition of data, or analysis and interpretation of data: JWEJ, GHG, HP, YE, NH and EEB. Drafting the article and revising it critically for intellectual content: JWEJ, GHG and EEB. Final approval of the version to be published: JWEJ, GHG, HP, YE, NH and EEB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Jocken, J., Goossens, G., Popeijus, H. et al. Contribution of lipase deficiency to mitochondrial dysfunction and insulin resistance in hMADS adipocytes. Int J Obes 40, 507–513 (2016). https://doi.org/10.1038/ijo.2015.211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.211
This article is cited by
-
The different effects of intramuscularly-injected lactate on white and brown adipose tissue in vivo
Molecular Biology Reports (2022)