Abstract
Background:
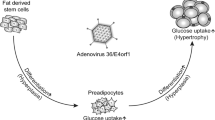
Various pathogens are implicated in the induction of obesity. Previous studies have confirmed that human adenovirus 36 (Ad36) is associated with increased adiposity, improved glycemic control and induction of inflammation. The Ad36-induced inflammation is reflected in the infiltration of macrophages into adipose tissue. However, the characteristics and role of adipose tissue macrophages (ATMs) and macrophage-secreted factors in virus-induced obesity (VIO) are unclear. Although insulin-like growth factor-1 (IGF-1) is involved in obesity metabolism, the contribution of IGF secreted by macrophages in VIO has not been studied.
Methods:
Four-week-old male mice were studied 1 week and 12 weeks after Ad36 infection for determining the characteristics of ATMs in VIO and diet-induced obesity (DIO). In addition, macrophage-specific IGF-1-deficient (MIKO) mice were used to study the involvement of IGF-1 in VIO.
Results:
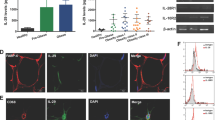
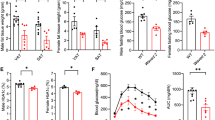
In the early stage of VIO (1 week after Ad36 infection), the M1 ATM sub-population increased, which increased the M1/M2 ratio, whereas DIO did not cause this change. In the late stage of VIO (12 weeks after Ad36 infection), the M1/M2 ratio did not change because the M1 and M2 ATM sub-populations increased to a similar extent, despite an increase in adiposity. By contrast, DIO increased the M1/M2 ratio. In addition, VIO in wild-type mice upregulated angiogenesis in adipose tissue and improved glycemic control. However, MIKO mice showed no increase in adiposity, angiogenesis, infiltration of macrophages into adipose tissue, or improvement in glycemic control after Ad36 infection.
Conclusions:
These data suggest that IGF-1 secreted by macrophages may contribute to hyperplasia and hypertrophy in adipose tissue by increasing angiogenesis, which helps to maintain the ‘adipose tissue robustness’.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gregor MF, Hotamisligil GS . Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29: 415–445.
Lumeng CN, Saltiel AR . Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121: 2111–2117.
Greenberg AS, Obin MS . Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83: 4615–4655.
Dandona P, Aljada A, Bandyopadhyay A . Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004; 25: 4–7.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 1481–1486.
Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378: 804–814.
Olefsky JM, Glass CK . Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246.
Ferrante AW . Macrophages, fat, and the emergence of immunometabolism. J Clin Invest 2013; 123: 4992–4993.
Wynn TA, Chawla A, Pollard JW . Macrophage biology in development, homeostasis and disease. Nature 2013; 496: 445–455.
Lewitt MS, Dent MS, Hall K . The insulin-like growth factor system in obesity, insulin resistance and type 2 diabetes mellitus. J Clin Med 2014; 3: 1561–1574.
Kahn SE, Hull RL, Utzschneider KM . Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846.
Berryman DE, Glad CA, List EO, Johannsson G . The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol 2013; 9: 346–356.
Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L . Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J 2011; 25: 358–369.
Boucher J, Mori MA, Lee KY, Smyth G, Liew CW, Macotela Y et al. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signaling. Nat Commun 2012; 3: 902.
Lumeng CN, Bodzin JL, Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184.
Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR . Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007; 56: 16–23.
Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR . Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008; 57: 3239–3246.
Martinez FO, Gordon S . The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014; 6: 13.
Sun K, Kusminski CM, Scherer PE . Adipose tissue remodeling and obesity. J Clin Invest 2011; 121: 2094–2101.
Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM . Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014; 17: 109–118.
Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obesity mice. Diabetes 2009; 58: 2574–2582.
Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem 2010; 285: 15333–15345.
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL . Increased adiposity in animals due to a human virus. Int J Obes 2000; 24: 989–996.
Na HN, Hong YM, Kim J, Kim HK, Jo I, Nam JH . Association between human adenovirus-36 and lipid disorders in Korean schoolchildren. Int J Obes 2010; 34: 89–93.
Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB et al. Human adenovirus-36 associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes 2005; 29: 281–286.
Trovato GM, Castro A, Tonzuso A, Garozzo A, Martines GF, Pirri C et al. Human obesity relationship with Ad36 adenovirus and insulin resistance. Int J Obes 2009; 33: 1402–1409.
Trovato GM, Martines GF, Garozzo A, Tonzuso A, Timpanaro R, Pirri C et al. Ad36 adipogenic adenovirus in human non-alcoholic fatty liver disease. Liver Int 2010; 30: 184–190.
Trovato GM, Martines GF, Trovato FM, Pirri C, Pace P, Garozzo A et al. Adenovirus-36 seropositivity enhances effects of nutritional intervention on obesity, bright liver, and insulin resistance. Dig Dis Sci 2012; 57: 535–544.
Wang ZQ, Cefalu WT, Zhang XH, Yu Y, Qin J, Son L et al. Human adenovirus type 36 enhances glucose uptake in diabetic and nondiabetic human skeletal muscle cells independent of insulin signaling. Diabetes 2008; 57: 1805–1813.
Na HN, Nam JH . Adenovirus 36 as an obesity agent maintains the obesity state by increasing MCP-1 and inducing inflammation. J Infect Dis 2012; 205: 914–922.
Cho A, Seok SH . Ethical guidelines for use of experimental animals in biomedical research. J Bacteriol Virol 2013; 43: 18–26.
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS . TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015–3025.
Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A2A receptor agonists and endotoxin. Am J Pathol 2002; 160: 2231–2244.
Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL et al. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity (Silver Spring, MD) 2006; 14: 1905–1913.
Krishnapuram R, Dhurandhar EJ, Dubuisson O, Kirk-Ballard H, Bajpeyi S, Butte N et al. Template to improve glycemic control without reducing adiposity or dietary fat. Am J Physiol Endocrinol Metab 2011; 300: E770–E789.
Rogers PM, Fusinski KA, Rathod MA, Loiler SA, Pasarica M, Shaw MK et al. Human adenovirus Ad-36 induces adipogenesis via its E4orf-1 gene. Int J Obes 2008; 32: 397–406.
Virtue S, Vidal-Puig A . Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta 2010; 1801: 338–349.
Laron Z . Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol 2001; 54: 311–316.
Huat TJ, Khan AA, Pati S, Mustafa Z, Abdullah JM, Jaafar H . IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci 2014; 15: 91.
Entingh-Pearsall A, Kahn CR . Differential roles of the insulin and insulin-like growth factor-1 (IGF-1) receptors in response to insulin and IGF-1. J Biol Chem 2004; 279: 38016–38024.
Cao Y . Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab 2013; 18: 478–489.
Acknowledgements
We thank Dr Anthony W Ferrante Jr from Columbia University for the gift of the MIKO mouse pair and Dr Yunhee Youm from Yale University for excellent guidance for the flow cytometry analysis. This work was supported by the Catholic University of Korea, Research Fund, 2015, by a grant from the Gyeonggi Regional Research Center of the Catholic University of Korea, by the Basic Science Research Program of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012R1A1A2039819) and by a grant from the Korean Healthcare Technology R&D project of the Ministry of Health and Welfare (HI13C0826 and 2015M3A9B5030116).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Park, S., Park, HL., Lee, SY. et al. Characteristics of adipose tissue macrophages and macrophage-derived insulin-like growth factor-1 in virus-induced obesity. Int J Obes 40, 460–470 (2016). https://doi.org/10.1038/ijo.2015.194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.194
This article is cited by
-
What we know and what we need to know about adenovirus 36-induced obesity
International Journal of Obesity (2020)
-
Growth hormone-releasing hormone is produced by adipocytes and regulates lipolysis through growth hormone receptor
International Journal of Obesity (2017)