Abstract
Background/Objective:
Genome-wide-association studies have identified numerous body mass index (BMI)-associated variants, but it is unclear how these relate to weight gain in adults at different ages.
Methods:
We examined the association of a genetic risk score (GRS), consisting of 31 BMI-associated variants, with an annual weight change (AWC) and a substantial weight gain (SWG) of 10% by comparing self-reported weight at 20 years (y) with baseline weight (mean: 58 y; s.d.: 8 y) in 21407 participants from the Malmö Diet and Cancer Study (MDCS), and comparing baseline weight to weight at follow-up (mean: 73 y; s.d.: 6 y) among 2673 participants. Association between GRS and AWG and SWG was replicated in 4327 GLACIER (Gene x Lifestyle interactions And Complex traits Involved in Elevated disease Risk) participants (mean: 45 y; s.d.: 7 y) with 10 y follow-up. Cohort-specific results were pooled by fixed-effect meta-analyses.
Results:
In MDCS, the GRS was associated with increased AWC (β: 0.003; s.e: 0.01; P: 7 × 10−8) and increased odds for SWG (odds ratio (OR) 1.01 (95% confidence interval (CI): 1.00, 1.02); P: 0.013) per risk-allele from age 20y, but unexpectedly with decreased AWC (β: −0.006; s.e: 0.002; P: 0.009) and decreased odds for SWG OR 0.96 (95% CI: 0.93, 0.98); P: 0.001) between baseline and follow-up. Effect estimates from age 20 y to baseline differed significantly from those from baseline to follow-up (P: 0.0002 for AWC and P: 0.0001 for SWG). Similar to MDCS, the GRS was associated with decreased odds for SWG OR 0.98 (95% CI: 0.96, 1.00); P: 0.029) from baseline to follow-up in GLACIER. In meta-analyses (n=7000), the GRS was associated with decreased AWC (β: −0.005; s.e.m. 0.002; P: 0.002) and decreased odds for SWG OR 0.97 (95% CI: 0.96, 0.99); P: 0.001) per risk-allele.
Conclusions:
Our results provide convincing evidence for a paradoxical inversed relationship between a high number of BMI-associated risk-alleles and less weight gain during and after middle-age, in contrast to the expected increased weight gain seen in younger age.
Similar content being viewed by others
Introduction
Global rates of obesity are increasing at an alarming rate and the worldwide prevalence of overweight and obesity has nearly doubled since 1980.1 The life expectancy of an overweight or obese individual is, on average, 10 years shorter than that of a normal-weight individual.2 In addition, overweight or obesity significantly increase the risk of numerous debilitating diseases, including diabetes, cardiovascular disease (CVD), hypertension and certain forms of cancer, such as postmenopausal breast cancer and endometrial, renal cell and colon cancers.3, 4 Excess body weight is not only associated with higher risks for numerous chronic diseases5 but also it often affects mobility, interferes with restful sleep and contributes to an overall lower quality of life when compared with normal-weight individuals.6, 7 In addition to body weight status, body weight gain irrespective of initial body mass index (BMI) has been associated with metabolic abnormalities.8, 9, 10
Factors that affect weight gain during adult life are not fully understood. Twin and family studies have indicated that ~40–70% of the inter-individual variance in BMI can be explained by genetic factors.11 Further, it is true that the increase in the prevalence of overweight and obesity worldwide can be attributed to changes in environmental factors, but the extent to which an individual is affected is also influenced by genetic factors.12 In the past 7 years, genome-wide association studies have identified 32 loci robustly associated with BMI13 and this list recently extended to 97 loci by the efforts of the GIANT consortium through the largest meta-analyses to date.14 Most of these studies were confined to middle-aged populations of European ancestry.
Despite the increased prevalence of overweight and obesity, life expectancy is continuing to rise in most countries.15 Studies have shown that factors associated with weight gain in middle age no longer carry the same associations after the age of 65 years, indicating that the causes and consequences of obesity may differ across a human’s lifespan.16
The purpose of this study was to investigate how genetic susceptibility to obesity, characterized as a genetic risk score (GRS) of 31 BMI-associated variants, affects annual weight change and substantial weight gain from young adulthood to middle age and later life.
Subjects and methods
Study populations
MDCS
The Malmö Diet and Cancer Study (MDCS) is a population-based cohort study based in southern Sweden; the initial study population consisted of 30 447 participants born between 1923 and 1950 who lived in the city of Malmö.17 During baseline examinations (1991–1996), which included the assessment of anthropometric variables and blood pressure measurements, participants provided blood samples and were also asked to complete a self-administered questionnaire on health and lifestyle-related factors, including information relating to any current and/or previous disease. Lack of Swedish language skills, and mental disability were the only exclusion criteria for recruitment. A detailed outline of the recruitment process and study procedures has been presented earlier.18
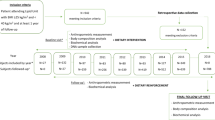
A schematic overview of inclusion and exclusion criteria for MDCS for the current study is presented in Figure 1. Out of the total 30 447 participants, 967 participants were excluded due to a lack of DNA data or crucial basic phenotype information, and an additional 1636 participants were excluded because of poor genotyping quality control (that is, <60% of the single-nucleotide polymorphisms (SNPs) were successfully genotyped). Questionnaire data relating to body weight at age 20 years was available for 80% (n=22 374) of the successfully genotyped participants. Further, we excluded 921 participants with diabetes at baseline and 46 participants for whom there was a mismatch between their baseline weight and their answers to two questions (at baseline) relating to weight at 20 years of age, that is, whether the weight from 20 years of age until the baseline examination was reported as stable or unstable, and whether it had decreased or increased. These exclusions left a total of 21 407 participants for the analysis in question. A random sub-cohort of 6103 MDCS participants who were alive and had not emigrated from Sweden (n=4924) were invited to a follow-up re-examination during 2007–2012. Of those invited, 3734 participants attended the follow-up re-examination, and of these, 2673 had access to information for self-reported weight at 20 years of age.
Schematic presentation of inclusion and exclusion criteria for participants of the MDCS in the current study. A total of 21 407 participants from the MDCS with self-reported data at baseline examination on weight at 20 years of age and 2673 participants with information on weight and BMI at three different time points (self-reported at 20 years age and measured weight and height at baseline and at follow-up) were included. The mean timespan from baseline to follow-up was 16.5 years (s.d.: 1.5 years) and mean time from 20 years of age until follow-up re-examination was 52.8 years (s.d.: 5.6 years).
GLACIER
The Gene x Lifestyle interactions And Complex traits Involved in Elevated disease Risk (GLACIER) Study is a population-based prospective cohort study of 19 547 participants nested within the ongoing Västerbotten health survey (VHU, Västerbottens Hälso Undersökning), which comprises more than 100 000 adults from the northern Swedish county of Västerbotten. Since 1985, all residents of the county of Västerbotten have been invited to visit their primary care center for a clinical examination in connection with their 40th, 50th and 60th birthdays. All participants underwent baseline examinations during 1985–2004. Data on clinical characteristics (age, sex, height and weight) were collected, and detailed assessments of lifestyle were made using a validated self-administered questionnaire.19, 20 A subgroup comprising 5010 GLACIER participants attended a 10-year follow-up examination between 1995 and 2007. All participants provided written informed consent as part of the VHU and ethical approval for the GLACIER Study was obtained from the regional ethical review board in Umeå. After excluding individuals with diabetes at baseline and individuals with inadequate genotype information (<60% successfully genotyped), 4327 GLACIER participants with prospective data were included in the present analysis.
SNP selection
We selected 32 SNPs in 32 loci identified by genome-wide association studies as being associated with BMI in European populations.13 One of the SNPs, rs4836133 representing the ZNF608 locus, was not successfully genotyped in MDCS and was excluded. Thus, in order to facilitate comparisons and the subsequent meta-analysis of results, the GRS was comprised of 31 SNPs, in both MDCS and GLACIER.
Genotyping
MDCS
The 31 BMI SNPs were genotyped using the Sequenom iPLEX platform, the Taqman allelic discrimination method, or the KASPar allelic discrimination method. Detailed information regarding selected SNPs, genotyping methods, quality control and related parameters are described in Supplementary Table 1. The average genotyping success rate was 97.2% and the distribution of genotype frequencies for each SNP were in Hardy–Weinberg equilibrium P>0.001 (Bonferroni-corrected P-value for 31 SNPs at level α=0.05).
GLACIER
Genotyping was performed by the Open Array SNP Genotyping System and the MetaboChip (Illumina Inc., San Diego, CA, USA) array, and SNP proxies were used for 12 out of the 31 SNPs. Information regarding SNPs used in GLACIER is provided in Supplementary Table 2. The average genotyping success rate was 96.0% and all SNPs were in Hardy–Weinberg equilibrium (P>0.001).
Genetic risk score calculation
Genotypes at each locus were coded as 0, 1 and 2, according to the number of BMI-increasing risk alleles and both weighted and unweighted GRSs were calculated in PLINK. To estimate the total BMI-increasing associated effects, the GRSs were calculated by summing up the number of BMI-increasing risk alleles and for weighted GRS each risk allele was weighted by their previously published effect sizes,13 while for the unweighted GRS the effect sizes were set equal to one. The weighted GRS was then rescaled to reflect the number of risk alleles, that is, each point of the GRS corresponded to one risk allele using a previously described method.21 There was no significant difference when model containing weighted GRS was tested for difference in the area under the receiver operating curve compared with the model containing unweighted GRS. As similar association patterns were observed with both GRSs, the results with weighted GRS are presented as it received a better fit than the unweighted GRS based on the seemingly unrelated estimation test (Supplementary Table 6).
Annual weight change calculation
In MDCS, we used information about weight at three time points, from which the average annual weight change was calculated from the difference between (i) self-reported weight at young age (age 20 years) and weight at the baseline examination (later middle age) (mean time period: 38 years; s.d.: 8 years); (ii) weight at baseline and the weight at follow-up examination (old age) (mean time period: 16.5 years; s.d.: 1.5 years). In GLACIER, annual weight change was calculated based on difference between weight at baseline (early middle age) and weight at the follow-up examination (late middle age) (mean time period: 9.9 years; s.d.: 0.5 years). For each time period, annual weight change was calculated by dividing the total weight change by the number of follow-up years. For the analyses of association between GRS and annual weight change during the follow-up stratified by weight loss or weight gain, the groups were defined as follows: compared with their weight at baseline, participants having lower weight at follow-up were defined as weight loss group (N=899 (MDCS) and N=1345 (GLACIER)) and participants having higher weight at follow-up were defined as weight gain group (N=1781 (MDCS) and N=2982 (GLACIER)).
Estimation of substantial weight gain
In MDCS, substantial weight gain was defined as (i) at baseline, having gained ⩾10% of self-reported weight at age of 20 years and (ii) at follow-up, having gained ⩾10% of baseline weight. Participants who gained, or lost <10% were defined as having stable weight. The number of participants who lost ⩾10% was small (⩽2.3%), and because excluding them from analysis did not materially influence the results, they were included in the stable weight group in all analyses. In GLACIER, weight was measured at baseline, and at follow-up examination ~10 years later. Substantial weight gain was defined as having gained ⩾10% of baseline weight at follow-up. Participants who lost ⩾10% weight between baseline, and follow-up (2.7%) were included in the weight-stable group in the analyses. For the analyses of association between GRS and substantial weight gain during the follow-up stratified by weight loss or weight gain during the follow-up time, the groups were defined as explained above for annual weight gain.
Mortality analyses in MDCS
In MDCS, total mortality and CVD mortality were examined. CVD was defined as fatal or non-fatal myocardial infarction or stroke, or death due to ischemic heart disease or stroke from the Swedish Hospital Discharge Register or Swedish National Cause of Death Register. Information on total mortality and CVD mortality during follow-up was retrieved by linking the 10-digit civil registration number with the Swedish National Cause of Death Register. Mortality was classified as attributable to cardiovascular causes when the main International Classification of Diseases code was ICD 9:390-459 or ICD 10:I00-I99 on the cause of death certificate. Follow-up extended until 31 December 2010. Mean±s.d. follow-up in analysis of mortality was 15.2±3.3 years. For analyses of mortality stratified by weight gain or weight stability before the baseline (from 20 years to baseline), the weight gain group was defined as having gained ⩾10% of self-reported weight at age of 20 years at baseline (N=15225) and participants who gained, or lost <10% were defined as having stable weight (N= 6182).
Statistical analyses
In MDCS, data were analyzed using SPSS version 20 (IBM Corp, Armonk, NY, USA), PLINK (version 1.07, http://pngu.mgh.harvard.edu/~purcell/plink/), and STATA (version 13, Stata Corp LP, College Station, TX, USA). Linear regression was used to estimate the effect (β) per unit increase of the GRS (equivalent to one BMI-increasing risk allele), per GRS quintile, or by comparing the extreme GRS quintiles on BMI (at age 20 years, baseline and follow-up) with annual weight change (from age 20 years to baseline, and from baseline to follow-up). Further, we used logistic regression to analyze the OR per increasing risk allele, or per GRS quintile, or by comparing extreme quintiles for substantial weight gain from age 20 years to baseline and from baseline to follow-up. In MDCS, we additionally performed similar analyses for waist circumference and waist-to-hip ratio (WHR), and annual change in these traits, as an attempt to investigate changes in body composition in relation to the BMI GRS. Analyses were adjusted for age, sex and follow-up time where applicable. To compare model coefficients, we used the seemingly unrelated estimation implementation in STATA (version 13, Stata Corp LP). This method allowed us to compare coefficients between models with partially overlapping, and thus dependent data. General linear model was used to test for interaction between GRS and weight gain and weight loss groups on annual weight change by including a multiplicative factor of the variables in the statistical model. We used Cox proportional hazard’s regression with time to follow-up until death, emigration or end of follow-up as time axis adjusted for age at baseline and sex to calculate the hazard ratios and 95% confidence intervals (95% CI) for GRS in relation to total and CVD-mortality. A two-sided P-value of <0.05 was considered statistically significant in the analyses with GRS. After correction for multiple testing, a P-value of <0.0016 (0.05/31) was considered significant in single SNP analyses. Replication analyses in GLACIER were performed using the same statistical models in SAS (version 9.3, SAS Institute Inc, Cary, NC, USA), STATA (version 12.1, Stata Corp LP) and PLINK (version 1.07). As no longitudinal data on waist or WHR existed in GLACIER, these analyses were only performed in MDCS.
Meta-analysis
To combine estimates from the MDCS and GLACIER studies, meta-analyses were performed in STATA (version 13, Stata Corp LP) using the metan command. Meta-analyses were performed using both fixed-effect and random-effect models. Results from the random-effect approach were not materially different from those from the fixed-effect approach; therefore, only the fixed-effect results are presented.
Results
The characteristics of the MDCS and GLACIER study cohorts at different ages, and time points are presented in Table 1.
Association between the GRS and BMI at different ages
In MDCS, the GRS was associated with higher BMI at all ages (P: 7 × 10−35; P: 2 × 10−34 and P: 0.005 at age 20 years, baseline and follow-up, respectively) with comparable effect sizes of 0.15, 0.23 and 0.19 kg m−2 per GRS quintile. Similarly, in GLACIER the GRS was associated with higher BMI both at baseline and at follow-up (P: 1 × 10−17 and P: 5 × 10−13, respectively) with somewhat higher effect sizes of 0.33 and 0.31, as compared with MDCS. Furthermore, GRS also associated with increased waist circumference (0.52 cm/GRS quintile, P: 1 × 10−29) and increased WHR (0.001/GRS quintile, P: 2 × 10−8) at baseline while no significant association of GRS was observed with these traits at follow-up.
Annual weight change and substantial weight gain from 20 years of age to baseline in MDCS (young age to late middle age)
In MDCS, the GRS was significantly associated with an increased annual weight gain per risk allele of the GRS (β: 0.003; s.e.m.: 0.001; P: 7 × 10−8), per GRS quintile (β: 0.007; s.e.m.: 0.001; P: 5 × 10−7), and for the highest versus lowest quintile (β: 0.032; s.e.m.: 0.006; P: 2.1 × 10−7) (Figure 2a). Among the individual SNPs, seven variants showed significant associations with increased annual weight gain but only the association of FTO rs1558902 (β: 0.013; s.e.m.: 0.003; P: 5 × 10−6) remained significant after the correction for multiple testing (Supplementary Table 3).
Association between the GRS of 31 BMI-associated variants with changes in weight at different periods of adult life. (a) Association of the GRS with annual weight change and (b) association of the GRS with substantial weight gain in MDCS and GLACIER. The lowermost results (overall) represent the meta-analyses of estimates in relation to annual weight change or substantial weight gain in n=7000 participants of MDCS and GLACIER from baseline to follow-up. MDCS, Malmö Diet and Cancer Study; GLACIER, Gene x Lifestyle interactions and complex traits involved in elevated disease risk.
In MDCS, 29% (n=6182) of the participants maintained a stable weight between age 20 years and baseline, and 71% (n=15225) had gained ⩾10% of weight at the time of the baseline visit. Similar to annual weight change, each unit increase of the GRS was associated with a 1% increased odds for substantial weight gain from age 20 years to baseline, and 3% increased odds per GRS quintile (P: 0.013 and P: 0.021, respectively). Participants in the highest GRS quintile have a 12% increased odds for substantial weight gain (P: 0.020) compared with participants in the lowest GRS quintile (Figure 2b). Among the individual SNPs, FTO rs1558902 (P: 0.047) and BDNF rs10767664 (P: 0.034) were associated with increased odds for substantial weight gain, whereas a reduced odds ratio (OR) was observed for TMEM160 rs3810291 (P: 0.008); none of these associations were significant after correction for multiple testing (Supplementary Table 3).
Annual weight change and substantial weight gain from baseline to follow-up in MDCS (late middle age to old age)
The GRS was associated with a weight reduction from baseline to follow-up with a per risk allele estimate of −0.006 kg (s.e: 0.002; P: 0.009), per quintile estimate of −0.018 kg (s.e: 0.006; P: 0.004), and an estimate of −0.062 kg (s.e: 0.03; P: 0.026) for the highest versus lowest quintile (Figure 2a). Among the individual SNPs, MC4R rs571312 (P: 0.042) showed a nominal significant association with decreasing weight gain, while QPCTL rs2287019 (P: 0.021) and FLJ35779 rs2112347 (P: 0.037) were significantly related to increased weight gain (Supplementary Table 4). Among the 2673 MDCS participants who attended the follow-up examination, 22% (n=587) had gained ⩾10% of weight, whereas 78% (n=2083) were weight stable. The GRS was associated with 4% decreased odds per risk allele and 10% decreased odds per GRS quintile (P: 0.001, and P: 0.002, respectively) for substantial weight gain from baseline to follow-up. Participants in the highest GRS quintile had 32% decreased odds for substantial weight gain (P: 0.011) compared with participants in the lowest GRS quintile (Figure 2b). Additional adjustment with baseline weight did not change the associations of GRS with substantial weight gain and annual weight change (Supplementary Table 7). Upon further stratification into weight gain and weight loss groups, the association of GRS with substantial weight gain remained unchanged but the inverse association between GRS and the annual weight gain was only significant among the weight loss group (Supplementary Table 8). However the associations did not differ significantly in the two groups (Pinteraction: 0.26). Among the individual SNPs, FTO rs1558902 (P: 0.008) and GNPDA2 rs10938397 (P: 0.037) showed a nominal significant association, while SLC39A8 rs13107325 (P: 0.001) showed significant association with decreased odds for substantial weight gain from baseline to follow-up (Supplementary Table 4).
As an attempt trying to further understand these results, we performed similar analyses for available longitudinal measures of body composition, that is, we tested association between GRS and annual changes in waist circumference and WHR in MDCS. These analyses revealed that similar to annual decrease in BMI, the GRS associated significantly with annual decrease in waist circumference. A similar trend was observed with annual WHR change but this association did not reach statistical significance (Supplementary Table 10).
Annual weight change and substantial weight gain from baseline to follow-up in GLACIER (early middle age to late middle age)
The GRS did not show any significant association with annual weight change in GLACIER (Figure 2a), and PTBP2 rs1555543 (P: 0.030) was the only variant that showed a nominal significant association with decreased annual weight gain from baseline to follow-up (Supplementary Table 4).
Among the 4327 participants in the GLACIER study, 21% (n=885) had gained ⩾10%, whereas 79% (n=3442) remained weight stable. The GRS was associated with 2% decreased odds per risk allele for a weight gain of ⩾10% from baseline to follow-up (P: 0.034) and showed a trend for 5% decreased odds per GRS quintile and 18% decreased odds when comparing the highest GRS quintile to the lowest GRS quintile from baseline to follow-up (P: 0.055 and P: 0.087, respectively) (Figure 2b). None of the variants showed any significant association with substantial weight gain from baseline to follow-up in GLACIER (Supplementary Table 4). Results did not change upon additional adjustment with baseline weight (Supplementary Table 7). Further stratification of individuals into weight gain and weight loss groups did not change the association of GRS with substantial weight gain from baseline to follow-up. However, for annual weight change we observed a significant inverse association between the GRS and annual weight change only in the weight loss group (Supplementary Table 8).
Comparison of effect estimates and OR in young to late middle age versus late middle age to old age in MDCS
In MDCS, we tested whether the association results of GRS or the individual SNPs with annual weight change or substantial weight gain differed significantly between the two time periods. For the association of GRS with annual weight change, the effect size (β) from age 20 years to baseline was significantly different from the effect size from baseline to follow-up (P: 0.0002 per risk allele and P: 0.0001 per quintile). For individual SNPs, it was only the effect estimates for FTO rs1558902 and MC4R rs571312 that were nominally significantly different between age 20 years to baseline, and from baseline to follow-up (Figure 3). Similarly, for the association of the GRS with substantial weight gain, the OR from age 20 years to baseline was significantly different from the OR from baseline to follow-up (P: 0.0001 per risk allele and P: 0.0002 per quintile). For individual SNPs, the OR for FTO rs1558902, GNPDA2 rs10938397, SH2B1 rs7359397 and TMEM160 rs3810291 was nominally significantly different, whereas the OR for SLC39A8 rs13107325 was significantly different when comparing the results from age 20 years to baseline and from baseline to follow-up (Figure 3).
Comparison of effect estimates for annual weight change and odds ratios for substantial weight gain for 31 BMI-associated variants, and their GRS from age 20 years to baseline with the same effect estimates and odds ratios from baseline to follow-up in MDCS. Black bars present the estimates (effect size or odds ratio) from age 20 years to baseline and the gray bars represent estimates from baseline to follow-up. SNPs are arranged based on the increasing order of estimates from baseline to follow-up. Striped bars indicate P-values for the comparison of estimates that were calculated using seemingly unrelated estimations.
Meta-analyses of MDCS and GLACIER for substantial weight gain from baseline to follow-up
In the meta-analyses of estimates from the MDCS and GLACIER studies comprising a total of 7000 participants, the GRS was significantly associated with decreased annual weight gain per risk allele (β: −0.005 kg; s.e.m.: 0.002; P: 0.002), per GRS quintile (β: −0.012 kg; s.e.m.: 0.005; P: 0.007), and when comparing the highest GRS quintile with the lowest (β: −0.052 kg; s.e.m.: 0.020; P: 0.011) from baseline to follow-up (Figure 2a). Similarly, a higher GRS was associated with less substantial weight gain between baseline and follow-up with 3% decreased odds (P: 0.001) per risk allele, 7% decreased odds per GRS quintile (P: 0.001), and 24% decreased odds when comparing the highest and lowest quintile of the score (P: 0.004) (Figure 2b).
FLJ35779 rs2112347 and PTBP2 rs1555543 were the only SNPs in the meta-analyses that showed a nominally significant association with increased and decreased annual weight gain from baseline to follow-up, respectively. FTO rs1558902 was the only SNP that showed nominally significantly decreased odds for a weight gain of ⩾10% from baseline to follow-up in the meta-analysis (OR 0·90 (95% CI: 0·83, 0·98), P: 0·019) (Supplementary Table 5).
Association of GRS with total mortality and CVD mortality in MDCS
To investigate if the observed inverse associations between GRS and weight gain in and after middle age could be explained by the GRS associating with mortality, we tested the association between GRS and total mortality and CVD mortality. In MDCS, 3879 participants died during a mean follow-up of 15 years, of which 1217 died of CVD. We did not find any significant association between GRS and total mortality (hazard ratios (95% CI): 1.01(1.00–1.02); P: 0.15) but observed a significant association with increased CVD mortality (hazard ratios (95% CI): 1.02(1.00–1.03); P: 0.029) (Supplementary Table 9). We further tested whether these associations differ between the individuals who kept their weight stable from 20 years to baseline, and those who substantially gained weight. Again, GRS did not significantly associate with total mortality but an increased CVD mortality was observed in particular among those in the highest GRS quintile compared to the lowest GRS quintile (hazard ratios (95% CI): 1.33(1.10–1.56); P:0.003), independent of whether the participants gained weight or not before middle age.
Discussion
This study, conducted in two prospective cohorts from Sweden, provides insights into how established genetic risk variants for adult BMI affect changes in weight across adult ages. The GRS was strongly associated with higher BMI at all adult ages. To clarify whether the same genetic variants that associate with increased BMI cross-sectionally also influence body weight gain over time, we investigated the annual weight change and substantial weight gain (⩾10%) between different adult age periods. In line with the cross-sectional results, a higher number of BMI-associated alleles associated with increased annual weight gain and substantial weight gain from young age to late middle age. In contrast, we made the paradoxical observation that during and after middle age a higher number of BMI-increasing risk alleles were associated with significantly decreased annual weight gain and less substantial weight gain.
In line with our study, earlier studies have indicated a possible discordance between genetic susceptibility for obesity in different age groups across lifespan. However, no previous studies have been able to demonstrate evidence for inverse and significantly different results for different adult age periods. One cross-sectional study that evaluated 10 BMI-associated SNPs in individuals of European and African American ancestry aged >65 years found that genetic variants associated with BMI in middle age may not hold the same association in old age.22 In addition, a recent Swedish study performed multifactorial analyses of factors affecting changes in BMI across the adult ages during a 65 year period and found evidence that a GRS comprising of 32 BMI-associated SNPs predicted an accelerated increase in BMI until middle age but not later in life.16 However, the design of that study was different from our study design, and whether the GRS may have had an inverse association with weight gain in older ages was not analyzed. As regards single SNP analysis, the association between FTO rs9939609 and obesity was observed to decline with older age, although effect estimates did not significantly differ by age.23 Interestingly, in our study, the FTO variant was significantly associated with decreased odds for substantial weight gain during and after middle age, and with significantly different effect estimates on weight change before and after middle age. In addition, Hardy et al.24 tested the variation in association between the FTO and MC4R variants with body size from birth to age 53 years and showed that the association reached peak strength at age 20 years and subsequently weakened during later adulthood.
In contrast to our findings, the Danish Inter99 study, in which the association between a GRS comprising 30 BMI SNPs and weight change was examined among 3982 individuals who were followed for 5 years from a mean age of 46.7 years, did not find any significant association.25 However, compared with MDCS, the Inter99 cohort was on average around 11 years younger at baseline, and its follow-up time was almost 10 years shorter. Further, although the ages of our GLACIER replication cohort, and the Inter99 cohort were comparable at baseline (45.2 vs 46.7), the follow-up time of GLACIER was almost twice that of Inter99, which could explain the different results. Indeed, the age and follow-up period of the Inter99 study potentially coincided with a time-point when the increased risk of weight gain as per the BMI-related GRS may had begun to shift toward decreased risk, thus resulting in no association. In line with the results in Inter99, we did not observe a significant association between the GRS and annual weight gain, but only with substantial weight gain, in the GLACIER cohort, which may indicate higher statistical power in the latter model.
It is difficult to explain the observed significantly inversed consequences of being a carrier of high numbers of BMI-associated alleles relating to weight gain until and after reaching middle age, and the current study is unable to provide such an explanation. It is important to note that in the current study the participants gained around 0.34 kg year−1 of weight from 20 year to baseline in MDCS while the weight gain from baseline to follow-up was 0.15 and 0.32 kg year−1 in MDCS and GLACIER, respectively. Thus, one might expect smaller effect estimates during or after middle age, due to less weight gain during the later time period as compared with weight gain earlier during adult life, but not the significantly inversed associations between the two time points. As an attempt trying to isolate the impact of genetic effects from non-genetic effects, we performed the analyses separately in individuals who gained weight (N= 1781 and 2982 for MDCS and GLACIER, respectively) or lost weight (N=889 and 1345 for MDCS and GLACIER, respectively) during the follow-up. The results were somewhat stronger for those that lost weight, which may indicate the possibility that non-genetic effects such as dieting and morbidity may have had some contribution to the observed results. In the sensitivity analyses, additional adjustment for the baseline weight did not affect the association of GRS with weight loss after the middle age, which decreases the possibility that this finding could be simply due to more obese people losing more weight in the older age. It is possible that the explanation lies in both physiological and genetic factors related to ageing. A strong body of evidence has demonstrated that with progressing age, loss of both bone and muscle tissue becomes evident along with increased body fat gain.26, 27 Loss of muscle mass is an important determinant of physical function and metabolic rate and results in the clinical hazards of obesity appearing at lower BMI levels in the elderly.28 In addition, it has been suggested that rapid weight gain before and during middle age has negative health consequences on par with having a consistently high BMI, or worse, and that such weight gain could ultimately lead to weight loss related to the negative effects of previous rapid weight gain, such as an elevated risk of obesity-related morbidity.29
Concerning mortality, we found evidence for association between the GRS and CVD mortality and these associations were similar irrespective of whether the individuals underwent a more pronounced weight gain until baseline or kept their weight stable. The associations with the CVD mortality were in particular stronger among those having higher number of BMI-risk alleles compared with those in the lowest quintile of GRS. Our study has several strengths, including a large sample size, repeated measurements of body weight and height, and a long follow-up period. Another important positive aspect of MDCS is that we were able to replicate our findings in another independent cohort from Sweden. Nonetheless, our study has some limitations that must be recognized, for example, in MDCS, weight at 20 years of age was self-reported, recalled by the participant several decades later. However, we did find that the GRS was strongly associated with self-reported BMI. Further, the GLACIER replication cohort was younger at baseline and its follow-up time was shorter, which probably affected the power of the replication analyses and was also reflected in the somewhat weaker results as compared to MDCS. Finally, it would have been informative to analyze associations between the GRS and the age-related changes in body composition such as fat mass, body fat percentage and lean body mass, as this might have facilitated the interpretation of our results. However, both cohorts lack sufficient data for such analysis. Nevertheless, longitudinal data on waist circumference and WHR was available in MDCS and both these phenotypes showed association patterns similar to those observed with annual weight change, indicating that at least part of the weight loss related to GRS is because of loss of abdominal fat.
In conclusion, our study suggests that the genetic susceptibility to obesity related to a GRS comprising 31 BMI-associated SNPs, is associated with BMI at all adult ages and with weight gain until middle age. However, our results indicate an inverse association with weight gain during and after middle age, which may suggest that the negative effects of weight gain before middle age may ultimately lead to age-related weight loss among individuals with high numbers of BMI-associated genetic variants. Whether the decreased risk of further weight gain during and after middle age among such individuals is a consequence of higher obesity-related morbidity, accelerated loss of muscle mass, or other age-related phenomena needs to be investigated in future studies.
References
Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr 2012; 10: 22.
Franco Sassi, Devaux M The Organisation for Economic Co-operation and Development (OECD); Obesity Update 2012. 2012.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M . Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371: 569–578.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH . The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9: 88.
CDC. Prevalence of obesity among adults with arthritis—United States, 2003–2009. MMRW Morb Mortal Wkly Rep 2011; 60: 509–513.
Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS . Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg 2008; 207: 928–934.
Fogarty AW, Glancy C, Jones S, Lewis SA, McKeever TM, Britton JR . A prospective study of weight change and systemic inflammation over 9 y. Am J Clin Nutr 2008; 87: 30–35.
Juntunen M, Niskanen L, Saarelainen J, Tuppurainen M, Saarikoski S, Honkanen R . Changes in body weight and onset of hypertension in perimenopausal women. J Hum Hypertens 2003; 17: 775–779.
Norman JE, Bild D, Lewis CE, Liu K, West DS . The impact of weight change on cardiovascular disease risk factors in young black and white adults: the CARDIA study. Int J Obes Relat Metab Disord 2003; 27: 369–376.
Maes HH, Neale MC, Eaves LJ . Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 1997; 27: 325–351.
Hebebrand J, Hinney A, Knoll N, Volckmar AL, Scherag A . Molecular genetic aspects of weight regulation. Dtsch Arztebl Int 2013; 110: 338–344.
Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010; 42: 937–948.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015; 518: 197–206.
Walls HL, Backholer K, Proietto J, McNeil JJ . Obesity and trends in life expectancy. J Obesity 2012; 2012: 107989.
Dahl AK, Reynolds CA, Fall T, Magnusson PKE, Pedersen NL . Multifactorial analysis of changes in body mass index across the adult life course: a study with 65 years of follow-up. Int J Obes 2014; 38: 1133–1141.
Berglund G, Elmstahl S, Janzon L, Larsson SA . The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med 1993; 233: 45–51.
Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M et al. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev 2001; 10: 489–499.
Hallmans G, Agren A, Johansson G, Johansson A, Stegmayr B, Jansson JH et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort—evaluation of risk factors and their interactions. Scand J Public Health Suppl 2003; 61: 18–24.
Renström F, Shungin D, Johansson I, the MI, Florez JC, Hallmans G et al. Genetic predisposition to long-term nondiabetic deteriorations in glucose homeostasis: ten-year follow-up of the GLACIER study. Diabetes 2011; 60: 345–354.
Cornelis MC, Qi L, Zhang C, Kraft P, Manson J, Cai T et al. Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med 2009; 150: 541–550.
Murphy RA, Nalls MA, Keller M, Garcia M, Kritchevsky SB, Tylavsky FA et al. Candidate gene association study of BMI-related loci, weight, and adiposity in old age. J Gerontol A Biol Sci Med Sci 2013; 68: 661–666.
Qi L, Kang K, Zhang C, van Dam RM, Kraft P, Hunter D et al. Fat mass–and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes 2008; 57: 3145–3151.
Hardy R, Wills AK, Wong A, Elks CE, Wareham NJ, Loos RJF et al. Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet 2010; 19: 545–552.
Sandholt CH, Allin KH, Toft U, Borglykke A, Ribel-Madsen R, Sparso T et al. The effect of GWAS identified BMI loci on changes in body weight among middle-aged danes during a five-year period. Obesity (Silver Spring) 2014; 22: 901–908.
Kaji H . Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care 2013; 16: 272–277.
Datta N . Muscle–bone and fat–bone interactions in regulating bone mass: do PTH and PTHrP play any role? Endocrine 2014; 47: 389–400.
Han TS, Tajar A, Lean ME . Obesity and weight management in the elderly. Br Med Bull 2011; 97: 169–196.
Zajacova A, Ailshire J . Body mass trajectories and mortality among older adults: a joint growth mixture-discrete-time survival analysis. Gerontologist 2014; 54: 221–231.
Acknowledgements
We thank Mrs Malin Svensson and Mrs Widet Tas for excellent technical assistance. We would also like to thank the staff and participants of the Malmö Diet and Cancer Study and the GLACIER study. This study was supported by the Swedish Research Council, the Swedish Heart and Lung Foundation, the Novo Nordic Foundation, the Swedish Diabetes Foundation and the Påhlsson Foundation, and by equipment grants from the Knut and Alice Wallenberg Foundation, the Region Skåne, Skåne University Hospital and the Linneus Foundation for the Lund University Diabetes Center. The funding sources had no role in study design, data collection, data analyses, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Rukh, G., Ahmad, S., Ericson, U. et al. Inverse relationship between a genetic risk score of 31 BMI loci and weight change before and after reaching middle age. Int J Obes 40, 252–259 (2016). https://doi.org/10.1038/ijo.2015.180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.180
This article is cited by
-
Systematic review of Mendelian randomization studies on risk of cancer
BMC Medicine (2022)
-
The timing of adiposity and changes in the life course on the risk of cancer
Cancer and Metastasis Reviews (2022)
-
Appetite disinhibition rather than hunger explains genetic effects on adult BMI trajectory
International Journal of Obesity (2021)
-
Genetic predisposition to obesity, restrained eating and changes in body weight: a population-based prospective study
International Journal of Obesity (2018)
-
Cardio-metabolic parameters are associated with genetic admixture estimates in a pediatric population from Colombia
BMC Genetics (2016)