Abstract
Introduction:
Thiazolidinediones (TZDs) enhanced body weight (BW) partially by increased adipogenesis and hyperphagia. Neuronal PPARγ knockout mice on high-fat diet (HFD) are leaner because of enhanced leptin response, although it could be secondary to their leanness. Thus, it still is an open question how TZDs may alter energy balance. Multiple factors regulate food intake (FI) and energy expenditure (EE), including anorexigenic hormones as insulin and leptin. Nonetheless, elevated hypothalamic AMPK activity increases FI and TZDs increase AMPK activity in muscle cells. Thus, the aim of the present study was to investigate whether Pioglitazone (PIO) treatment alters hypothalamic insulin and leptin action/signaling, AMPK phosphorylation, and whether these alterations may be implicated in the regulation of FI and EE.
Methods:
Swiss mice on HFD (2 months) received PIO (25 mg kg−1 per day-gavage) or vehicle for 14 days. AMPK and AdipoR1 were inhibited via Intracerebroventricular injections using Compound C (CompC) and small interference RNA (siRNA), respectively. Western blot, real-time PCR and CLAMS were done.
Results:
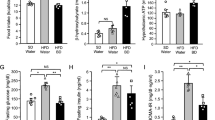
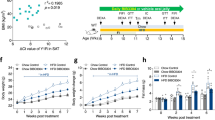
PIO treatment increased BW, adiposity, FI, NPY mRNA and decreased POMC mRNA expression and EE in HFD mice. Despite higher adiposity, PIO treatment improved insulin sensitivity, glucose tolerance, decreased insulin and increased adiponectin serum levels. This result was associated with, improved insulin and leptin action/signaling, decreased α2AMPKSer491 phosphorylation and elevated Acetyl-CoA carboxylase and AMPKThr172 phosphorylation in hypothalamus. The inhibition of hypothalamic AMPK with CompC was associated with decreased adiposity, FI, NPY mRNA and EE in PIO-treated mice. The reduced expression of hypothalamic AdipoR1 with siRNA concomitantly with PIO treatment reverted PIO induced obesity development, suggesting that adiponectin may be involved in this effect.
Conclusions:
These results demonstrated that PIO, despite improving insulin/leptin action in hypothalamus, increases FI and decreases EE, partially, by activating hypothalamic adiponectin/AdipoR1/AMPK axis. Suggesting a novel mechanism in the hypothalamus by which TZDs increase BW.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378: 804–814.
de Almeida-Pititto B, Dias ML, de Moraes AC, Ferreira SR, Franco DR, Eliaschewitz FG . Type 2 diabetes in Brazil: epidemiology and management. Diabetes Metab Syndr Obes 2015; 8: 17–28.
Forouhi NG, Wareham NJ . Epidemiology of diabetes. Medicine 2014; 42: 698–702.
Desouza CV, Shivaswamy V . Pioglitazone in the treatment of type 2 diabetes: safety and efficacy review. Clin Med Insights Endocrinol Diabetes 2010; 3: 43–51.
Govindan J, Evans M . Pioglitazone in clinical practice: where are we now? Diabetes Ther 2012; 3: 1–8.
Lehrke M, Lazar MA . The many faces of PPARgamma. Cell 2005; 123: 993–999.
Shimizu H, Tsuchiya T, Sato N, Shimomura Y, Kobayashi I, Mori M . Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care 1998; 21: 1470–1474.
Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD et al. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 2009; 150: 707–712.
Tontonoz P, Hu E, Spiegelman BM . Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994; 79: 1147–1156.
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999; 4: 611–617.
Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes 2003; 52: 910–917.
Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR . Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med 1998; 339: 953–959.
Larsen PJ, Jensen PB, Sorensen RV, Larsen LK, Vrang N, Wulff EM et al. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes 2003; 52: 2249–2259.
Pickavance LC, Buckingham RE, Wilding JP . Insulin-sensitizing action of rosiglitazone is enhanced by preventing hyperphagia. Diabetes Obes Metab 2001; 3: 171–180.
Wang Q, Dryden S, Frankish HM, Bing C, Pickavance L, Hopkins D et al. Increased feeding in fatty Zucker rats by the thiazolidinedione BRL 49653 (rosiglitazone) and the possible involvement of leptin and hypothalamic neuropeptide Y. Br J Pharmacol 1997; 122: 1405–1410.
Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ . A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat Med 2011; 17: 623–626.
Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G et al. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med 2011; 17: 618–622.
Belgardt BF, Bruning JC . CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 2010; 1212: 97–113.
Stark R, Ashley SE, Andrews ZB . AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol Cell Endocrinol 2013; 366: 215–223.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004; 428: 569–574.
Carling D, Thornton C, Woods A, Sanders MJ . AMP-activated protein kinase: new regulation, new roles? Biochem J 2012; 445: 11–27.
Caricilli AM, Penteado E, de Abreu LL, Quaresma PG, Santos AC, Guadagnini D et al. Topiramate treatment improves hypothalamic insulin and leptin signaling and action and reduces obesity in mice. Endocrinology 2012; 153: 4401–4411.
Castro G, C Areias MF, Weissmann L, Quaresma PG, Katashima CK, Saad MJ et al. Diet-induced obesity induces endoplasmic reticulum stress and insulin resistance in the amygdala of rats. FEBS Open Bio 2013; 3: 443–449.
Hardie DG . The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci 2004; 117: 5479–5487.
Carling D . AMP-activated protein kinase: balancing the scales. Biochimie 2005; 87: 87–91.
Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB . p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab 2012; 16: 104–112.
Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem 2006; 281: 5335–5340.
Hurley RL, Barré LK, Anderson KA, Kemp BE, Means AR, Witters LA et al. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 2006; 281: 36662–36672.
Yamauchi T, Kadowaki T . Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes 2008; 32: S13–S18.
Kadowaki T, Yamauchi T, Kubota N . The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 2008; 582: 74–80.
Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007; 6: 55–68.
Minokoshi Y, Shiuchi T, Lee S, Suzuki A, Okamoto S . Role of hypothalamic AMP-kinase in food intake regulation. Nutrition 2008; 24: 786–790.
Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A et al. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol 2009; 200: 93–105.
Segal-Lieberman G, Trombly DJ, Juthani V, Wang X, Maratos-Flier E . NPY ablation in C57BL/6 mice leads to mild obesity and to an impaired refeeding response to fasting. Am J Physiol Endocrinol Metab 2003; 284: E1131–E1139.
Kageyama H, Takenoya F, Hirako S, Wada N, Kintaka Y, Inoue S et al. Neuronal circuits involving neuropeptide Y in hypothalamic arcuate nucleus-mediated feeding regulation. Neuropeptides 2012; 46: 285–289.
Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L . Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 2002; 5: 566–572.
Munzberg H, Flier JS, Bjorbaek C . Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004; 145: 4880–4889.
Tripathy D, Daniele G, Fiorentino TV, Perez-Cadena Z, Chavez-Velasquez A, Kamath S et al. Pioglitazone improves glucose metabolism and modulates skeletal muscle TIMP-3-TACE dyad in type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled, mechanistic study. Diabetologia 2013; 56: 2153–2163.
Monroy A, Kamath S, Chavez AO, Centonze VE, Veerasamy M, Barrentine A et al. Impaired regulation of the TNF-alpha converting enzyme/tissue inhibitor of metalloproteinase 3 proteolytic system in skeletal muscle of obese type 2 diabetic patients: a new mechanism of insulin resistance in humans. Diabetologia 2009; 52: 2169–2181.
Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J et al. The inflammatory status score including IL-6, TNF-alpha, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol 2014; 51: 123–131.
Swanson CR Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE et al. The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation 2011; 8: 91.
Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med 2011; 17: 618–622.
Ryan KK Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ . A role for central nervous system PPAR-γ in the regulation of energy balance. Nat Med 2011; 17: 623–626.
Cariou B, Charbonnel B, Staels B . Thiazolidinediones and PPARgamma agonists: time for a reassessment. Trends Endocrinol Metab 2012; 23: 205–215.
Acknowledgements
This work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) Sao Paulo, Brazil: Auxílio Regular 2012/10338-6 and CEPID 2013/07607-8. CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico): INCT (Instituto Nacional Ciência e Tecnologia em Obesidade e Diabetes) 573856/2008-7 and UNIVERSAL 481084/2013-4.
We thank L. Janeri, J. Pinheiro and Andrey dos Santos (Department of Internal Medicine, UNICAMP, Campinas, Sao Paulo, Brazil) for their technical assistance.
The present work received financial support from FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo): Auxílio Regular 2012/10338-6 and CEPID 2013/07607-8, São Paulo, Brazil and from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) Universal (481084/2013-4) and INCT (Instituto Nacional Ciência e Tecnologia de Obesidade e Diabetes) (573856/2008-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Quaresma, P., Reencober, N., Zanotto, T. et al. Pioglitazone treatment increases food intake and decreases energy expenditure partially via hypothalamic adiponectin/adipoR1/AMPK pathway. Int J Obes 40, 138–146 (2016). https://doi.org/10.1038/ijo.2015.134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.134
This article is cited by
-
Leveraging Human Genetics to Identify Safety Signals Prior to Drug Marketing Approval and Clinical Use
Drug Safety (2020)
-
Short-term exposure to air pollution (PM2.5) induces hypothalamic inflammation, and long-term leads to leptin resistance and obesity via Tlr4/Ikbke in mice
Scientific Reports (2020)
-
Cdc2-like kinase 2 in the hypothalamus is necessary to maintain energy homeostasis
International Journal of Obesity (2017)